The Use of β-Blockers in Heart Failure with Reduced Ejection Fraction
Abstract
:1. Introduction
2. Use of β-Blockers in HFrEF: Pathophysiology and Clinical Pharmacology
- (1)
- Nonselective β-blockers with similar β1 and β2 activity (none of the β-blockers belonging to this class is indicated for HFrEF);
- (2)
- β1-selective with a higher affinity for β1-adrenoreceptors (metoprolol, bisoprolol, and nebivolol), preferred in patients with chronic obstructive pulmonary disease or mild asthma (nebivolol also facilitates nitric oxide release and is preferred in patients with arterial hypertension);
- (3)
- β-blockers with additional α-1-adrenoreceptor antagonism and consequent peripheral vasodilation (carvedilol), preferred in patients with hypertension or documented higher peripheral vascular resistance.
3. Evidence Supporting the Use of β-Blockers in HFrEF
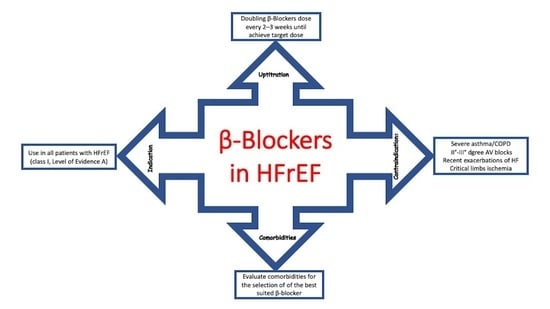
4. Optimizing the Use of β-Blockers in HFrEF: A Practical Approach
- (1)
- Peripheral congestion: β-Blockers should not be initiated in patients with moderate to severe fluid retention [44]. Because HFrEF is a progressive disease, it is likely that, during its course, many patients will develop signs and symptoms related to fluid retention. The initial approach to these patients is based on fluid management, often increasing the dose or adding a second diuretic (sequential nephron blockade) [45]. In the presence of congestion, initiation of therapy or increase in β-blocker dosage should be deferred until euvolemia is achieved [46]. In general, discontinuation or dose reduction of a β-blocker is not indicated in the presence of congestion unless it is associated with hypoperfusion (in case of a cold and wet patient).
- (2)
- Asymptomatic hypotension: This is common in patients with HFrEF and is not a contraindication to β-blocker therapy [47]. It is essential to consider whether hypotension is caused by an inadequate preload related to aggressive use of diuretics or vasodilators [48]. In such cases, it may be necessary to reduce or suspend these therapies.
- (3)
- Symptomatic bradycardia: β-Blockers may be used in patients with asymptomatic, mild bradycardia, particularly when the heart rate increases with exercise [49]. The possibility of drug interactions that may lower the heart rate (e.g., digoxin and amiodarone) should also be considered [50]. Given the substantial benefits of β-blockers in HFrEF, asymptomatic bradycardia during β-blocker therapy is not a reason to discontinue it, and cardiac pacing should be considered on an individual basis [51].
5. Use of β-Blockers in Patients with Heart Failure and Comorbidities: A Practical Approach
5.1. Asthma and Chronic Obstructive Pulmonary Disease (COPD)
5.2. Diabetes Mellitus (DM)
5.3. Atrial Fibrillation (AF)
5.4. Peripheral Artery Disease
6. Knowledge Gaps and Outstanding Research Questions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Savarese, G.; Lund, L.H. Global public health burden of heart failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation 2018, 137, e67. [Google Scholar] [CrossRef]
- Komajda, M.; Böhm, M.; Borer, J.S.; Ford, I.; Tavazzi, L.; Pannaux, M.; Swedberg, K. Incremental benefit of drug therapies for chronic heart failure with reduced ejection fraction: A network meta-analysis. Eur. J. Heart Fail. 2018, 20, 1315–1322. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J. Systolic heart failure. N. Engl. J. Med. 2010, 362, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Teerlink, J.R. Heart failure. Lancet 2017, 390, 1981–1995. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar]
- Maddox, T.M.; Januzzi, J.L., Jr.; Allen, L.A.; Breathett, K.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; Masoudi, F.A.; et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure with Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 772–810. [Google Scholar]
- McDonald, M.; Virani, S.; Chan, M.; Ducharme, A.; Ezekowitz, J.A.; Giannetti, N.; Heckman, G.A.; Howlett, J.G.; Koshman, S.L.; Lepage, S.; et al. CCS/CHFS Heart Failure Guidelines Update: Defining a New Pharmacologic Standard of Care for Heart Failure With Reduced Ejection Fraction. Can. J. Cardiol. 2021, 37, 531–546. [Google Scholar] [CrossRef]
- Barrese, V.; Taglialatela, M. New advances in beta-blocker therapy in heart failure. Front. Physiol. 2013, 4, 323. [Google Scholar] [CrossRef] [Green Version]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753. [Google Scholar] [CrossRef]
- Berthelot, E.; Eicher, J.; Salvat, M.; Seronde, M.; de Groote, P. Medical inertia in the optimization of heart failure treatment after discharge and its relationship to outcome. Health Care Curr. Rev. 2018, 6, 2. [Google Scholar]
- Loop, M.S.; Van Dyke, M.K.; Chen, L.; Safford, M.M.; Kilgore, M.L.; Brown, T.M.; Durant, R.W.; Levitan, E.B. Low Utilization of Beta-Blockers Among Medicare Beneficiaries Hospitalized for Heart Failure with Reduced Ejection Fraction. J. Card. Fail. 2018, 16, 31102–31107. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L.; Bristow, M.R. Mechanisms and models in heart failure: The biomechanical model and beyond. Circulation 2005, 111, 2837–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudd, J.O.; Kass, D.A. Tackling heart failure in the twenty-first century. Nature 2008, 451, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Karayannis, G.; Giamouzis, G.; Skoularigis, J.; Louridas, G.; Butler, J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J. Am. Coll. Cardiol. 2009, 54, 1747–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armour, J.A. Cardiac neuronal hierarchy in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R262–R271. [Google Scholar] [CrossRef] [Green Version]
- Brodde, O.E. Beta-adrenoceptors in cardiac disease. Pharmacol. Ther. 1993, 60, 405–430. [Google Scholar] [CrossRef]
- Lohse, M.J.; Engelhardt, S.; Eschenhagen, T. What is the role of beta-adrenergic signaling in heart failure? Circ. Res. 2003, 93, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.J.; Engelhardt, S.; Danner, S.; Böhm, M. Mechanisms of beta-adrenergic receptor desensitization: From molecular biology to heart failure. Basic Res. Cardiol. 1996, 91, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Brodde, O.E.; Hillemann, S.; Kunde, K.; Vogelsang, M.; Zerkowski, H.R. Receptor systems affecting force of contraction in the human heart and their alterations in chronic heart failure. J. Heart Lung Transplant. 1992, 11, S164–S174. [Google Scholar]
- Bristow, M.R.; Hershberger, R.E.; Port, J.D.; Gilbert, E.M.; Sandoval, A.; Rasmussen, R.; Cates, A.E.; Feldman, A.M. Beta-adrenergic pathways in nonfailing and failing human ventricular myocardium. Circulation 1990, 82, I12–I25. [Google Scholar] [PubMed]
- Liaudet, L.; Calderari, B.; Pacher, P. Pathophysiological mechanisms of catecholamine and cocaine-mediated cardiotoxicity. Heart Fail. Rev. 2014, 19, 815–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouri, F.; Findji, L.; Mediani, O.; Mougenot, N.; Hanoun, N.; Le Naour, G.; Hamon, M.; Lechat, P. Toxic cardiac effects of catecholamines: Role of beta-adrenoceptor downregulation. Eur. J. Pharmacol. 2002, 456, 69–75. [Google Scholar] [CrossRef]
- Lefkowitz, R.J.; Rockman, H.A.; Koch, W.J. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation 2000, 101, 1634–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouzamondo, A.; Hulot, J.S.; Sanchez, P.; Cucherat, M.; Lechat, P. Beta-blocker treatment in heart failure. Fundam. Clin. Pharmacol. 2001, 15, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R. Treatment of chronic heart failure with β-adrenergic receptor antagonists: A convergence of receptor pharmacology and clinical cardiology. Circ. Res. 2011, 109, 1176–1194. [Google Scholar] [CrossRef] [Green Version]
- Abraham, W.T. Beta-blockers: The new standard of therapy for mild heart failure. Arch. Intern. Med. 2000, 160, 1237–1247. [Google Scholar] [CrossRef] [Green Version]
- Kitai, T.; Tang, W.H. Pathophysiologic insights into heart rate reduction in heart failure: Implications in the use of beta-blockers and ivabradine. Curr. Treat. Options Cardiovasc. Med. 2016, 18, 13. [Google Scholar] [CrossRef]
- Grandi, E.; Ripplinger, C.M. Antiarrhythmic mechanisms of beta blocker therapy. Pharmacol. Res. 2019, 146, 104274. [Google Scholar] [CrossRef]
- Martínez-Milla, J.; Raposeiras-Roubín, S.; Pascual-Figal, D.A.; Ibáñez, B. Role of Beta-blockers in Cardiovascular Disease in 2019. Rev. Esp. Cardiol. (Engl. Ed.) 2019, 72, 844–852. [Google Scholar] [CrossRef]
- Doughty, R.N.; White, H.D. Carvedilol: Use in chronic heart failure. Expert Rev. Cardiovasc. Ther. 2007, 5, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A. Stunned and Hibernating Myocardium: Where Are We Nearly 4 Decades Later? J. Am. Heart Assoc. 2020, 9, e015502. [Google Scholar] [CrossRef]
- Bristow, M. Antiadrenergic therapy of chronic heart failure: Surprises and new opportunities. Circulation 2003, 107, 1100–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waagstein, F.; Bristow, M.R.; Swedberg, K.; Camerini, F.; Fowler, M.B.; Silver, M.A.; Gilbert, E.M.; Johnson, M.R.; Goss, F.G.; Hjalmarson, A. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group. Lancet 1993, 342, 1441–1446. [Google Scholar] [CrossRef]
- Merit-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999, 353, 2001–2007. [Google Scholar] [CrossRef]
- Poole-Wilson, P.A.; Swedberg, K.; Cleland, J.G.; Di Lenarda, A.; Hanrath, P.; Komajda, M.; Lubsen, J.; Lutiger, B.; Metra, M.; Remme, W.J.; et al. Carvedilol or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol or Metoprolol European Trial (COMET): Randomized controlled trial. Lancet 2003, 362, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Packer, M.; Bristow, M.R.; Cohn, J.N.; Colucci, W.S.; Fowler, M.B.; Gilbert, E.M.; Shusterman, N.H. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N. Engl. J. Med. 1996, 334, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Dargie, H.J. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: The CAPRICORN randomized trial. Lancet 2001, 357, 1385–1390. [Google Scholar]
- Fowler, M.B. Carvedilol prospective randomized cumulative survival (COPERNICUS) trial: Carvedilol in severe heart failure. Am. J. Cardiol. 2004, 93, 35–39. [Google Scholar] [CrossRef] [PubMed]
- The Cardiac Insufficiency Bisoprolol Study (CIBIS). CIBIS Investigators and Committees. A randomized trial of beta-blockade in heart failure. Circulation 1994, 90, 1765–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CIBIS-II Investigators. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomized trial. Lancet 1999, 353, 9–13. [Google Scholar] [CrossRef]
- Flather, M.D.; Shibata, M.C.; Coats, A.J.; Van Veldhuisen, D.J.; Parkhomenko, A.; Borbola, J.; Cohen-Solal, A.; Dumitrascu, D.; Ferrari, R.; Lechat, P.; et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur. Heart J. 2005, 26, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, J. Heart failure drug treatment: The fantastic four. Eur. Heart J. 2021, 42, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Colucci, W.S.; Swedberg, K. Beta-blockers in chronic heart failure. Circulation 2003, 107, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure with congestion—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, J.M. Beta-blockers continue to surprise us. Eur. Heart J. 2000, 21, 354–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, D.T.; Hebert, P.R.; Coffey, C.S.; Curtis, J.P.; Foody, J.M.; Sedrakyan, A.; Krumholz, H.M. Adverse effects of beta-blocker therapy for patients with heart failure: A quantitative overview of randomized trials. Arch. Intern. Med. 2004, 164, 1389–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inamdar, A.A.; Inamdar, A.C. Heart Failure: Diagnosis, Management and Utilization. J. Clin. Med. 2016, 5, 62. [Google Scholar] [CrossRef]
- Ramahi, T.M. Beta blocker therapy for chronic heart failure. Am. Fam. Physician 2000, 62, 2267–2274. [Google Scholar] [PubMed]
- Blaufarb, I.; Pfeifer, T.M.; Frishman, W.H. beta-blockers. Drug interactions of clinical significance. Drug Saf. 1995, 13, 359–370. [Google Scholar] [CrossRef]
- Hoppe, U.C. Beta-blocker induced bradycardia-should we pace? Eur. J. Heart Fail. 2004, 6, 449–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correale, M.; Paolillo, S.; Mercurio, V.; Limongelli, G.; Barillà, F.; Ruocco, G.; Palazzuoli, A.; Scrutinio, D.; Lagioia, R.; Lombardi, C.; et al. Comorbidities in chronic heart failure: An update from Italian Society of Cardiology (SIC) Working Group on Heart Failure. Eur. J. Intern. Med. 2020, 71, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straw, S.; McGinlay, M.; Relton, S.D.; Koshy, A.O.; Gierula, J.; Paton, M.F.; Drozd, M.; Lowry, J.E.; Cole, C.; Cubbon, R.M.; et al. Effect of disease-modifying agents and their association with mortality in multi-morbid patients with heart failure with reduced ejection fraction. ESC. Heart Fail. 2020, 7, 3859–3870. [Google Scholar] [CrossRef]
- Güder, G.; Brenner, S.; Störk, S.; Hoes, A.; Rutten, F.H. Chronic obstructive pulmonary disease in heart failure: Accurate diagnosis and treatment. Eur. J. Heart Fail. 2014, 16, 1273–1282. [Google Scholar] [CrossRef]
- Iversen, K.K.; Kjaergaard, J.; Akkan, D.; Kober, L.; Torp-Pedersen, C.; Hassager, C.; Vestbo, J.; Kjoller, E. Chronic obstructive pulmonary disease in patients admitted with heart failure. J. Intern. Med. 2008, 264, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.; Lourenco, P.; Lopes, R.; Azevedo, A.; Bettencourt, P. Chronic obstructive pulmonary disease in heart failure. Prevalence, therapeutic and prognostic implications. Am. Heart J. 2008, 155, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, W.; Voors, A.A.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; van der Harst, P.; Hillege, H.L.; Lang, C.C.; Ter Maaten, J.M.; et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: A prospective European study. Eur. Heart J. 2017, 38, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, A.; Macdonald, P.S.; Keogh, A.M.; Kotlyar, E.; Mellemkjaer, S.; Coleman, C.F.; Elsik, M.; Krum, H.; Hayward, C.S. Differences between beta-blockers in patients with chronic heart failure and chronic obstructive pulmonary disease: A randomized crossover trial. J. Am. Coll. Cardiol. 2010, 55, 1780–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, D.R.; Lipworth, B.J.; Donnan, P.T.; Jackson, C.; Guthrie, B. Respiratory effect of beta-blockers in people with asthma and cardiovascular disease: Population-based nested case control study. BMC Med. 2017, 15, 18. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.Y.; Tseng, P.T.; Wu, Y.C.; Tu, Y.K.; Stubbs, B.; Su, K.P.; Matsuoka, Y.J.; Hsu, C.W.; Lin, C.H.; Chen, Y.W.; et al. Do beta-adrenergic blocking agents increase asthma exacerbation? A network meta-analysis of randomized controlled trials. Sci. Rep. 2021, 11, 452. [Google Scholar] [CrossRef]
- Cruickshank, J.M. Beta-blockers and diabetes: The bad guys come good. Cardiovasc. Drugs Ther. 2002, 16, 457–470. [Google Scholar] [CrossRef]
- Mills, G.A.; Horn, J.R. Beta-blockers and glucose control. Drug Intell. Clin. Pharm. 1985, 19, 246–251. [Google Scholar] [CrossRef]
- Sanon, V.P.; Sanon, S.; Kanakia, R.; Yu, H.; Araj, F.; Oliveros, R.; Chilton, R. Hypoglycemia from a cardiologist’s perspective. Clin. Cardiol. 2014, 37, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.J.; Vos, T.; Gilbert, R.E.; Krum, H. Are beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta-analysis of large-scale clinical trials. Am. Heart J. 2003, 146, 848–853. [Google Scholar] [CrossRef]
- Wai, B.; Kearney, L.G.; Hare, D.L.; Ord, M.; Burrell, L.M.; Srivastava, P.M. Beta blocker use in subjects with type 2 diabetes mellitus and systolic heart failure does not worsen glycaemic control. Cardiovasc. Diabetol. 2012, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisel, W.H.; Stevenson, L.W. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 2003, 91, 2–8. [Google Scholar] [CrossRef]
- Kotecha, D.; Holmes, J.; Krum, H.; Altman, D.G.; Manzano, L.; Cleland, J.G.; Lip, G.Y.; Coats, A.J.; Andersson, B.; Kirchhof, P.; et al. Beta-Blockers in Heart Failure Collaborative Group. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: An individual-patient data meta-analysis. Lancet 2014, 384, 2235–2243. [Google Scholar] [CrossRef] [Green Version]
- Cadrin-Tourigny, J.; Shohoudi, A.; Roy, D.; Talajic, M.; Tadros, R.; Mondésert, B.; Dyrda, K.; Rivard, L.; Andrade, J.G.; Macle, L.; et al. Decreased Mortality with Beta-Blockers in Patients with Heart Failure and Coexisting Atrial Fibrillation: An AF-CHF Substudy. JACC. Heart Fail. 2017, 5, 99–106. [Google Scholar] [CrossRef]
- Baher, A.; Marrouche, N.F. Treatment of Atrial Fibrillation in Patients with Co-existing Heart Failure and Reduced Ejection Fraction: Time to Revisit the Management Guidelines? Arrhythm. Electrophysiol. Rev. 2018, 7, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Frishman, W.H. Beta adrenergic receptor blockers: Adverse effects and drug interactions. Hypertension 1988, 11, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breckenridge, A. Which beta-blocker? BMJ 1983, 286, 1085–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thulesius, O. Beta-adrenergic blockade and vasospasm. Acta. Med. Scand. 1979, 625, 41–43. [Google Scholar] [CrossRef]
- Fogoros, R.N. Exacerbation of intermittent claudication by propranolol. NEJM 1980, 302, 1089. [Google Scholar] [PubMed]
- Paravastu, S.C.; Mendonca, D.A.; Da Silva, A. Beta blockers for peripheral arterial disease. Cochrane Database Syst. Rev. 2013, 2013. [Google Scholar] [CrossRef]
- Kotecha, D.; Gill, S.K.; Flather, M.D.; Holmes, J.; Packer, M.; Rosano, G.; Böhm, M.; McMurray, J.J.V.; Wikstrand, J.; Anker, S.D.; et al. Beta-Blockers in Heart Failure Collaborative Group. Impact of Renal Impairment on Beta-Blocker Efficacy in Patients with Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 2893–2904. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Du Fay Lavallaz, J.; Benson, L.; Savarese, G.; Dahlström, U.; Lund, L.H. Association Between β-Blockers and Outcomes in Heart Failure with Preserved Ejection Fraction: Current Insights From the SwedeHF Registry. J. Card. Fail. 2021. [Google Scholar] [CrossRef]

| Type of Mechanisms |
|---|
| Imbalance of myocardial oxygen supply/demand |
| Calcium overload, with subsequent phosphorylation of multiple Ca(2+)-cycling proteins |
| Increased oxidative stress due the increased formation of “aminochromes” |
| Coronary spasm |
| Depletion of energy stores |
| Increased mitochondrial permeability |
| Trial | Year | Type of β-Blockers | n° of Patients | Inclusion Criteria | Effects on Mortality |
|---|---|---|---|---|---|
| CIBIS | 1994 | Bisoprolol | 641 | LVEF < 40%, NYHA class III-V | No significant difference in mortality between the two groups |
| MERIT HF | 1999 | Metoprolol | 3991 | LVEF < 40%, NYHA class II-IV | 34% relative risk reduction in all-cause mortality |
| CIBIS II | 1999 | Bisoprolol | 2647 | LVEF < 35%, NYHA class III-IV | 34% relative risk reduction in all-cause mortality |
| CAPRICORN | 2001 | Carvedilol | 1959 | Previous AMI and LVEF < 40% | 23% relative risk reduction in all-cause mortality |
| COPERNICUS | 2001 | Carvedilol | 2289 | LVEF < 25% and NYHA class III-IV | 31% relative risk reduction in all-cause mortality |
| COMET | 2003 | Metoprolol vs Carvedilolo | 2309 | LVEF < 35% and NYHA class II-IV | 17% relative risk reduction in all-cause mortality in carvedilol group |
| SENIORS | 2005 | Nebivolol | 2128 | LVEF < 35%, NYHA class II-IV, age > 7o years | No significant difference in mortality between the two groups |
| Clinical Scenario | β-Blockers |
|---|---|
| Hypertension | Carvedilol, nebivolol |
| Asthma and Chronic Obstructive Pulmonary Disease | Bisoprolol, nebivolol |
| Diabetes mellitus | Carvedilol, bisoprolol |
| Atrial fibrillation | Metoprolol, bisoprolol |
| Peripheral Artery Disease | Carvedilol, nebivolol |
| Hypercholesterolemia | Carvedilol |
| Hyperthyroidism | Metoprolol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masarone, D.; Martucci, M.L.; Errigo, V.; Pacileo, G. The Use of β-Blockers in Heart Failure with Reduced Ejection Fraction. J. Cardiovasc. Dev. Dis. 2021, 8, 101. https://doi.org/10.3390/jcdd8090101
Masarone D, Martucci ML, Errigo V, Pacileo G. The Use of β-Blockers in Heart Failure with Reduced Ejection Fraction. Journal of Cardiovascular Development and Disease. 2021; 8(9):101. https://doi.org/10.3390/jcdd8090101
Chicago/Turabian StyleMasarone, Daniele, Maria Luigia Martucci, Vittoria Errigo, and Giuseppe Pacileo. 2021. "The Use of β-Blockers in Heart Failure with Reduced Ejection Fraction" Journal of Cardiovascular Development and Disease 8, no. 9: 101. https://doi.org/10.3390/jcdd8090101