Influence of Ag on the Properties of Ca0.9Yb0.1MnO3 Sintered Ceramics
Abstract
:1. Introduction
2. Materials and Methods
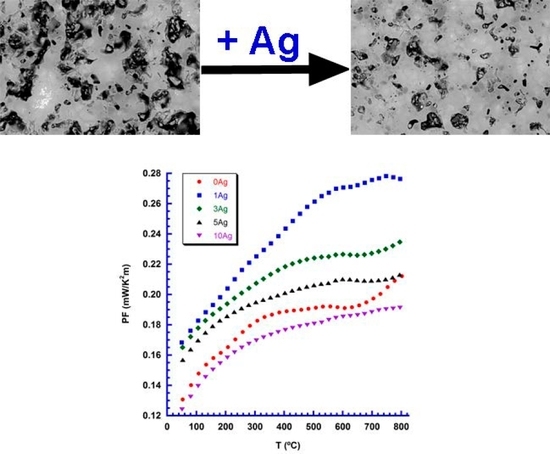
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elsheikh, M.H.; Shnawah, D.A.; Sabri, M.F.M.; Said, S.B.M.; Hassan, M.H.; Bashir, M.B.A.; Mohamad, M. A review on thermoelectric renewable energy: Principle parameters that affect their performance. Renew. Sustain. Energy Rev. 2014, 30, 337–355. [Google Scholar] [CrossRef]
- Kleinke, H. New bulk materials for thermoelectric power generation: Clathrates and complex antimonides. Chem. Mater. 2010, 22, 604–611. [Google Scholar] [CrossRef]
- Vaqueiro, P.; Sobany, G.G.; Powell, A.V.; Knight, K.S. Structure and thermoelectric properties of the ordered skutterudite CoGe1.5Te1.5. J. Solid State Chem. 2006, 179, 2047–2053. [Google Scholar] [CrossRef]
- Rowe, D.M. General Principles and basic Considerations. In Thermolectrics Handbook: Macro to Nano, 1st ed.; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1-3–1-7. [Google Scholar]
- Sun, N.; Dong, S.T.; Zhang, B.B.; Chen, Y.B.; Zhou, J.; Zhang, S.T.; Gu, Z.B.; Yao, S.H.; Chen, Y.F. Intrinsically modified thermoelectric performance of alkaline-earth isovalently substituted [Bi2AE2O4][CoO2]y single crystals. J. Appl. Phys. 2013, 114, 043705. [Google Scholar] [CrossRef]
- Sotelo, A.; Rasekh, S.; Torres, M.A.; Bosque, P.; Madre, M.A.; Diez, J.C. Effect of synthesis methods on the Ca3Co4O9 thermoelectric ceramic performances. J. Solid State Chem. 2015, 221, 247–254. [Google Scholar] [CrossRef]
- Delorme, F.; Martin, C.F.; Marudhachalam, P.; Ovono Ovono, D.; Guzman, G. Effect of Ca substitution by Sr on the thermoelectric properties of Ca3Co4O9 ceramics. J. Alloy. Compd. 2011, 509, 2311–2315. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Su, W.-B.; Liu, J.; Zhou, Y.-C.; Li, J.; Zhang, X.; Du, Y.; Wang, C.-L. Effects of Dy and Yb co-doping on thermoelectric properties of CaMnO3 ceramics. Ceram. Int. 2015, 41, 1535–1539. [Google Scholar] [CrossRef]
- Sotelo, A.; Torres, M.A.; Madre, M.A.; Diez, J.C. Effect of synthesis process on the densification; microstructure; and electrical properties of Ca0.9Yb0.1MnO3 ceramics. Int. J. Appl. Ceram. Technol. 2017, 14, 1190–1196. [Google Scholar] [CrossRef]
- Kovalevsky, A.V.; Aguirre, M.H.; Populoh, S.; Patricio, S.G.; Ferreira, N.M.; Mikhalev, S.M.; Fagg, D.P.; Weidenkaff, A.; Frade, J.R. Designing strontium titanate-based thermoelectrics: insight into defect chemistry mechanisms. J. Mater. Chem. A 2017, 5, 3909–3922. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Yan, X.; Huo, D.; Li, X.; Li, J.-G.; Ding, X. Fabrication and thermoelectric properties of highly textured Ca9Co12O28 ceramic. J. Alloy. Compd. 2014, 582, 294–298. [Google Scholar] [CrossRef]
- Noudem, J.G.; Kenfaui, D.; Chateigner, D.; Gomina, M. Toward the enhancement of thermoelectric properties of lamellar Ca3Co4O9 by edge-free spark plasma texturing. Scr. Mater. 2012, 66, 258–260. [Google Scholar] [CrossRef]
- Torres, M.A.; Garcia, G.; Urrutibeascoa, I.; Madre, M.A.; Diez, J.C.; Sotelo, A. Fast preparation route to high-performances textured Sr-doped Ca3Co4O9 thermoelectric materials through precursor powder modification. Sci. China Mater. 2018. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Wang, C.L.; Su, W.B.; Liu, J.; Wang, H.C.; Li, J.C.; Li, Y.; Zhai, J.Z.; Zhang, Y.C.; Mei, L.M. Electrical properties of Dy3+/Na+ Co-doped oxide thermoelectric [Ca1-x(Na1/2Dy1/2)x]MnO3 ceramics. J. Alloy. Compd. 2016, 680, 129–132. [Google Scholar] [CrossRef]
- Mouyane, M.; Itaalit, B.; Bernard, J.; Houivet, D.; Noudem, J.G. Flash combustion synthesis of electron doped-CaMnO3 thermoelectric oxides. Powder Technol. 2014, 264, 71–77. [Google Scholar] [CrossRef]
- Wang, H.C.; Wang, C.L. Thermoelectric properties of Yb-doped La0.1Sr0.9TiO3 ceramics at high temperature. Ceram. Int. 2013, 39, 941–946. [Google Scholar] [CrossRef]
- Srivastava, D.; Norman, C.; Azough, F.; Schafer, M.C.; Guilmeau, E.; Kepaptsoglou, D.; Ramasse, Q.M.; Nicotrad, G.; Freer, R. Tuning the thermoelectric properties of A-site deficient SrTiO3 ceramics by vacancies and carrier concentration. Phys. Chem. Chem. Phys. 2016, 18, 26475–26486. [Google Scholar] [CrossRef] [PubMed]
- Flahaut, D.; Mihara, T.; Funahashi, R.; Nabeshima, N.; Lee, K.; Ohta, H.; Koumoto, K. Thermoelectrical properties of A-site substituted Ca1−xRexMnO3 system. J. Appl. Phys. 2006, 100, 084911. [Google Scholar] [CrossRef]
- Sotelo, A.; Depriester, M.; Torres, M.A.; Sahraoui, A.H.; Madre, M.A.; Diez, J.C. Effect of simultaneous K, and Yb substitution for Ca on the microstructural and thermoelectric characteristics of CaMnO3 ceramics. Ceram. Int. 2018, 44, 12697–12701. [Google Scholar] [CrossRef]
- Kabir, R.; Wang, D.; Zhang, T.; Tian, R.; Donelson, R.; Tan, T.T.; Li, S. Tunable thermoelectric properties of Ca0.9Yb0.1MnO3 through controlling the particle size via ball mill processing. Ceram. Int. 2014, 40, 16701–16706. [Google Scholar] [CrossRef]
- Zhang, B.; Chang, A.; Zhao, Q.; Ye, H.; Wu, Y. Synthesis and Thermoelectric Properties of Yb-doped Ca0.9−xYbxLa0.1MnO3 Ceramics. J. Electron. Mater. 2014, 43, 4048–4055. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C. Synthesis of Dy doped Yb0.1Ca0.9MnO3 ceramics with a high relative density and their thermoelectric properties. Mater. Res. Bull. 2012, 47, 2252–2256. [Google Scholar] [CrossRef]
- Boldrin, D.; Boldrin, P.; Ruiz-Trejo, E.; Cohen, L.F. Recovery of the intrinsic thermoelectric properties of CaMn0.98Nb0.02O3 in 2-terminal geometry using Ag infiltration. Acta Mater. 2017, 133, 68–72. [Google Scholar] [CrossRef]
- Costa, F.M.; Ferreira, N.M.; Rasekh, S.; Fernandes, A.J.S.; Torres, M.A.; Madre, M.A.; Diez, J.C.; Sotelo, A. Very large superconducting currents induced by growth tailoring. Cryst. Growth Des. 2015, 15, 2094–2101. [Google Scholar] [CrossRef]
- Kosuga, A.; Urata, S.; Kurosaki, K.; Yamanaka, S.; Funahashi, R. Mechanical Properties of Ca0.9Yb0.1MnO3/Ag Composites for n-Type Legs of Thermoelectric Oxide Devices. Jpn. J. Appl. Phys. 2008, 47, 6399–6403. [Google Scholar] [CrossRef]
- Kahraman, F.; Madre, M.A.; Rasekh, S.; Salvador, C.; Bosque, P.; Torres, M.A.; Diez, J.C.; Sotelo, A. Enhancement of mechanical and thermoelectric properties of Ca3Co4O9 by Ag addition. J. Eur. Ceram. Soc. 2015, 35, 3835–3841. [Google Scholar] [CrossRef]
- Joo, J.; Singh, J.P.; Warzynski, T.; Grow, A.; Poeppel, R.B. Role of silver addition on mechanical and superconducting properties of high-Tc superconductors. Appl. Supercond. 1994, 2, 401–410. [Google Scholar] [CrossRef]
- Lide, D.R. Physical Constants of Inorganic Compounds. In CRC Handbook of Chemistry and Physics, 90th ed.; Lide, D.R., Ed.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2009; pp. 4-44–4-101. [Google Scholar]
- Wang, Y.; Sui, Y.; Cheng, J.; Wang, X.; Lu, Z.; Su, W. High temperature metal-insulator transition induced by rare-earth doping in perovskite CaMnO3. J. Phys. Chem. C 2009, 113, 12509–12516. [Google Scholar] [CrossRef]
- Poeppelmeier, K.R.; Leonowicz, M.E.; Longo, J.M. CaMnO2.5 and Ca2MnO3.5: New oxygen-defect perovskite-type oxides. J. Solid State Chem. 1982, 44, 89–98. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, Y.; Chang, P.; Ye, L. Morphology-controlled synthesis of monodisperse silver spheres via a solvothermal method. J. Alloy. Compd. 2011, 509, 5381–5387. [Google Scholar] [CrossRef]
- Mikami, M.; Ando, N.; Funahashi, R. The effect of Ag addition on electrical properties of the thermoelectric compound Ca3Co4O9. J. Solid State Chem. 2005, 178, 2186–2190. [Google Scholar] [CrossRef]
- Maignan, A.; Martin, C.; Damay, F.; Raveau, B. Transition from a paramagnetic metallic to a cluster glass metallic state in electron-doped perovskite manganites. Phys. Rev. B 1998, 58, 2758–2763. [Google Scholar] [CrossRef]
- Madre, M.A.; Costa, F.M.; Ferreira, N.M.; Costa, S.I.R.; Rasekh, S.; Torres, M.A.; Diez, J.C.; Amaral, V.S.; Amaral, J.S.; Sotelo, A. High thermoelectric performance in Bi2−xPbxBa2Co2Oy promoted by directional growth and annealing. J. Eur. Ceram. Soc. 2016, 36, 67–74. [Google Scholar] [CrossRef]
- Kabir, R.; Tian, R.; Zhang, T.; Donelson, R.; Tan, T.T.; Li, S. Role of Bi doping in thermoelectric properties of CaMnO3. J. Alloy. Compd. 2015, 628, 347–351. [Google Scholar] [CrossRef]






| x | Density (g/cm3) | Standard Error | Relative Density (%) |
|---|---|---|---|
| 0 | 3.74 | 0.05 | 75 |
| 1 | 4.14 | 0.03 | 83 |
| 3 | 4.37 | 0.04 | 86 |
| 5 | 4.64 | 0.02 | 91 |
| 10 | 4.53 | 0.03 | 86 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotelo, A.; Torres, M.A.; Madre, M.A.; Diez, J.C. Influence of Ag on the Properties of Ca0.9Yb0.1MnO3 Sintered Ceramics. Materials 2018, 11, 2503. https://doi.org/10.3390/ma11122503
Sotelo A, Torres MA, Madre MA, Diez JC. Influence of Ag on the Properties of Ca0.9Yb0.1MnO3 Sintered Ceramics. Materials. 2018; 11(12):2503. https://doi.org/10.3390/ma11122503
Chicago/Turabian StyleSotelo, Andrés, Miguel A. Torres, María A. Madre, and Juan C. Diez. 2018. "Influence of Ag on the Properties of Ca0.9Yb0.1MnO3 Sintered Ceramics" Materials 11, no. 12: 2503. https://doi.org/10.3390/ma11122503