Behavior of Molybdenum–Vanadium Mixed Oxides in Selective Oxidation and Disproportionation of Toluene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Characterization of the Catalysts
2.3. Catalytic Tests
3. Results and Discussions
3.1. Catalysts Characterization
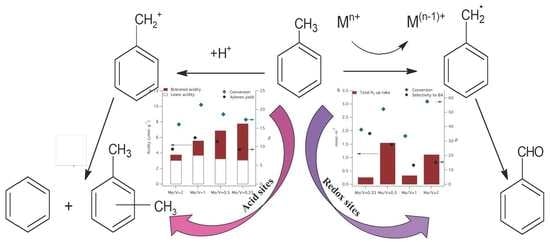
3.2. Toluene Disproportionation
3.3. Toluene Selective Oxidation
3.4. General Assessments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ji, Y.-J.; Zhang, B.; Xu, L.; Wu, H.; Peng, H.; Chen, L.; Liu, Y.; Wu, P. Core/shell-structured Al-MWW@B-MWW zeolites for shape-selective toluene disproportionation to para-xylene. J. Catal. 2011, 283, 168–177. [Google Scholar] [CrossRef]
- Tsai, T.-C.; Chen, W.-H.; Lai, C.S.; Liu, S.-B.; Wang, I.; Ku, C.S. Kinetics of toluene disproportionation over fresh and coked H-mordenite. Catal. Today 2004, 97, 297–302. [Google Scholar] [CrossRef]
- Uguina, M.A.; Sotelo, J.L.; Serrano, D.P. Roles of ZSM-5 modifier agents in selective toluene disproportionation. Can. J. Chem. Eng. 1993, 71, 558–563. [Google Scholar] [CrossRef]
- Kunieda, T.; Kim, J.-H.; Niwa, M. Source of Selectivity of p-Xylene Formation in the Toluene Disproportionation over HZSM-5 Zeolites. J. Catal. 1999, 188, 431–433. [Google Scholar] [CrossRef]
- Xiong, Y.; Rodewald, P.G.; Chang, C.D. On the Mechanism of Toluene Disproportionation in a Zeolite Environment. J. Am. Chem. Soc. 1995, 117, 9427–9431. [Google Scholar] [CrossRef]
- Fong, Y.Y.; Abdullah, A.Z.; Ahmad, A.L.; Bhatia, S. Development of functionalized zeolite membrane and its potential role as reactor combined separator for para-xylene production from xylene isomers. Chem. Eng. J. 2008, 139, 172–193. [Google Scholar] [CrossRef]
- Gallego, E.M.; Portilla, M.T.; Paris, C.; León-Escamilla, A.; Boronat, M.; Moliner, M.; Corma, A. “Abinitio” synthesis of zeolites for preestablished catalytic reactions. Science 2017, 355, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Cejka, J.; Wichterlová, B.; Eder-Mirth, G.; Lercher, J.A. Decisive role of transport rate of products for zeolite para-selectivity: Effect of coke deposition and external surface silylation on activity and selectivity of HZSM-5 in alkylation of toluene. Zeolites 1996, 17, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Suganuma, S.; Nakamura, K.; Okuda, A.; Katada, N. Enhancement of catalytic activity for toluene disproportionation by loading Lewis acidic nickel species on ZSM-5 zeolite. Mol. Catal. 2017, 435, 110–117. [Google Scholar] [CrossRef]
- Mitra, B.; Kunzru, D. Enhancing p-xylene productivity for disproportionation of toluene in microstructured reactors. Chem. Eng. Process. Process Intensif. 2013, 64, 48–56. [Google Scholar] [CrossRef]
- Tsai, T.-C. Reactivation of acidic sites in mordenite used in toluene disproportionation. Appl. Catal. A 2006, 301, 292–298. [Google Scholar] [CrossRef]
- Hino, M.; Kurashige, M.; Matsuhashi, H.; Arata, K. A solid acid of tungsta-niobia more active than aluminosilicates for decompositions of cumene, ethylbenzene, and toluene. Appl. Catal. A 2006, 310, 190–193. [Google Scholar] [CrossRef]
- Wang, D.; Osmundsen, C.M.; Taarning, E.; Dumesic, J.A. Selective Production of Aromatics from Alkylfurans over Solid Acid Catalysts. ChemCatChem 2013, 5, 2044–2050. [Google Scholar] [CrossRef]
- Williams, C.L.; Chang, C.-C.; Do, P.; Nikbin, N.; Caratzoulas, S.; Vlachos, D.G.; Lobo, R.F.; Fan, W.; Dauenhauer, P.J. Cycloaddition of Biomass-Derived Furans for Catalytic Production of Renewable p-Xylene. ACS Catal. 2012, 2, 935–939. [Google Scholar] [CrossRef]
- Huang, M.M.; Howe, R.F. Reactions of Methanol and Toluene over Molybdenum Zeolites. In Studies in Surface Science and Catalysis; Bibby, D.M., Chang, C.D., Howe, R.F., Yurchak, S., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 36, pp. 207–211. [Google Scholar]
- Al-Khattaf, S.; Musilová-Pavlačková, Z.; Ali, M.A.; Čejka, J. Comparison of Activity and Selectivity of SSZ-33 Based Catalyst with other Zeolites in Toluene Disproportionation. Top. Catal. 2008, 52, 140. [Google Scholar] [CrossRef]
- Kesavan, L.; Tiruvalam, R.; Rahim, M.H.A.; bin Saiman, M.I.; Enache, D.I.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Taylor, S.H.; Knight, D.W.; et al. Solvent-Free Oxidation of Primary Carbon-Hydrogen Bonds in Toluene Using Au-Pd Alloy Nanoparticles. Science 2011, 331, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Losada, P.J.; Heckl, I.; Bertok, B.; Friedler, F.; García-Ojeda, J.; Argoti, A. Process Network Synthesis for Benzaldehyde Production: P-graph Approach. Chem. Eng. Trans. 2015, 45, 1369–1374. [Google Scholar]
- Xue, M.; Chen, H.; Zhang, H.; Auroux, A.; Shen, J. Preparation and characterization of V-Ag-O catalysts for the selective oxidation of toluene. Appl. Catal. A 2010, 379, 7–14. [Google Scholar] [CrossRef]
- Ge, J.; Xue, M.; Sun, Q.; Auroux, A.; Shen, J. Surface acidic and redox properties of V-Zr-O catalysts for the selective oxidation of toluene to benzaldehyde. J. Mol. Catal. A Chem. 2007, 278, 209–214. [Google Scholar] [CrossRef]
- Bautista, F.M.; Campelo, J.M.; Luna, D.; Luque, J.; Marinas, J.M. Gas-phase selective oxidation of toluene on TiO2–sepiolite supported vanadium oxides: Influence of vanadium loading on conversion and product selectivities. Catal. Today 2007, 128, 183–190. [Google Scholar] [CrossRef]
- Solsona, B.; Dejoz, A.; Garcia, T.; Concepción, P.; Nieto, J.M.L.; Vázquez, M.I.; Navarro, M.T. Molybdenum–vanadium supported on mesoporous alumina catalysts for the oxidative dehydrogenation of ethane. Catal. Today 2006, 117, 228–233. [Google Scholar] [CrossRef]
- Heracleous, E.; Machli, M.; Lemonidou, A.A.; Vasalos, I.A. Oxidative dehydrogenation of ethane and propane over vanadia and molybdena supported catalysts. J. Mol. Catal. A Chem. 2005, 232, 29–39. [Google Scholar] [CrossRef]
- Liu, R.; Wang, T.; Cai, D.; Jin, Y. Highly Efficient Production of Acrylic Acid by Sequential Dehydration and Oxidation of Glycerol. Ind. Eng. Chem. Res. 2014, 53, 8667–8674. [Google Scholar] [CrossRef]
- Nedyalkova, R.; Ilieva, L.; Bernard, M.C.; Hugot-Le Goff, A.; Andreeva, D. Gold supported catalysts on titania and ceria, promoted by vanadia or molybdena for complete benzene oxidation. Mater. Chem. Phys. 2009, 116, 214–218. [Google Scholar] [CrossRef]
- Tokarz-Sobieraj, R.; Witko, M.; Gryboś, R. Reduction and re-oxidation of molybdena and vanadia: DFT cluster model studies. Catal. Today 2005, 99, 241–253. [Google Scholar] [CrossRef]
- Vishnetskaya, M.V.; Tomskiy, I.S. Role of Singlet Oxygen in the Oxidation of Toluene on Vanadium Molybdenum Catalytic Systems. Chem. Sustain. Dev. 2011, 19, 321–325. [Google Scholar]
- Li, Y.; Liu, T.; Li, T.; Peng, X. Hydrothermal fabrication of controlled morphologies of MoO3 with CTAB: Structure and growth. Mater. Lett. 2015, 140, 48–50. [Google Scholar] [CrossRef]
- Huang, P.-R.; He, Y.; Cao, C.; Lu, Z.-H. Impact of lattice distortion and electron doping on α-MoO3 electronic structure. Sci. Rep. 2014, 4, 7131. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.C.; Ravindranadh, K.; Kasturi, A.; Shekhawat, M.S. Structural Stoichiometry and Phase Transitions of MoO3 Thin Films for Solid State Microbatteries. Res. J. Recent Sci. 2013, 2, 67–73. [Google Scholar]
- Giebeler, L.; Kampe, P.; Wirth, A.; Adams, A.H.; Kunert, J.; Fuess, H.; Vogel, H. Structural changes of vanadium–molybdenum–tungsten mixed oxide catalysts during the selective oxidation of acrolein to acrylic acid. J. Mol. Catal. A Chem. 2006, 259, 309–318. [Google Scholar] [CrossRef]
- Adams, A.H.; Haaß, F.; Buhrmester, T.; Kunert, J.; Ott, J.; Vogel, H.; Fuess, H. Structure and reaction studies on vanadium molybdenum mixed oxides. J. Mol. Catal. A Chem. 2004, 216, 67–74. [Google Scholar] [CrossRef]
- Zou, J.Y.; Schrader, G.L. Deposition of multiphase molybdate thin films by reactive sputtering. Thin Solid Films 1998, 324, 52–62. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Zhang, Z. Growth of [010] oriented α-MoO3 nanorods by pulsed electron beam deposition. Appl. Phys. Lett. 2011, 99, 223104. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, T.; Reddy, K.R.; Kumar, C.P.; Murthy, M.R.V.S.; Chary, K.V.R. Characterization and reactivity of molybdenum oxide catalysts supported on zirconia. Appl. Catal. A 2001, 211, 189–201. [Google Scholar] [CrossRef]
- Wang, D.; Jangjou, Y.; Liu, Y.; Sharma, M.K.; Luo, J.; Li, J.; Kamasamudram, K.; Epling, W.S. A comparison of hydrothermal aging effects on NH3-SCR of NOx over Cu-SSZ-13 and Cu-SAPO-34 catalysts. Appl. Catal. B 2015, 165, 438–445. [Google Scholar] [CrossRef]
- Ciambelli, P.; Sannino, D.; Palma, V.; Vaiano, V.; Eloy, P.; Dury, F.; Gaigneaux, E.M. Tuning the selectivity of MoOx supported catalysts for cyclohexane photo oxidehydrogenation. Catal. Today 2007, 128, 251–257. [Google Scholar] [CrossRef]
- Vakros, J.; Lycourghiotis, A.; Voyiatzis, G.A.; Siokou, A.; Kordulis, C. CoMo/Al2O3-SiO2 catalysts prepared by co-equilibrium deposition filtration: Characterization and catalytic behavior for the hydrodesulphurization of thiophene. Appl. Catal. B 2010, 96, 496–507. [Google Scholar] [CrossRef]
- Duan, A.; Wan, G.; Zhao, Z.; Xu, C.; Zheng, Y.; Zhang, Y.; Dou, T.; Bao, X.; Chung, K. Characterization and activity of Mo supported catalysts for diesel deep hydrodesulphurization. Catal. Today 2007, 119, 13–18. [Google Scholar] [CrossRef]
- Higashimoto, S.; Matsuoka, M.; Yamashita, H.; Anpo, M.; Kitao, O.; Hidaka, H.; Che, M.; Giamello, E. Effect of the Si/Al Ratio on the Local Structure of V Oxide/ZSM-5 Catalysts Prepared by Solid-State Reaction and Their Photocatalytic Reactivity for the Decomposition of NO in the Absence and Presence of Propane. J. Phys. Chem. B 2000, 104, 10288–10292. [Google Scholar] [CrossRef]
- Mitran, G.; Pavel, O.-D.; Marcu, I.-C. Molybdena–vanadia supported on alumina: Effective catalysts for the esterification reaction of acetic acid with n-butanol. J. Mol. Catal. A Chem. 2013, 370, 104–110. [Google Scholar] [CrossRef]
- Ghampson, I.T.; Pecchi, G.; Fierro, J.L.G.; Videla, A.; Escalona, N. Catalytic hydrodeoxygenation of anisole over Re-MoOx/TiO2 and Re-VOx/TiO2 catalysts. Appl. Catal. B 2017, 208, 60–74. [Google Scholar] [CrossRef]
- Benomar, S.; Massó, A.; Solsona, B.; Issaadi, R.; López Nieto, M.J. Vanadium Supported on Alumina and/or Zirconia Catalysts for the Selective Transformation of Ethane and Methanol. Catalysts 2018, 8, 126. [Google Scholar] [CrossRef]
- Deo, G.; Wachs, I.E. Reactivity of Supported Vanadium Oxide Catalysts: The Partial Oxidation of Methanol. J. Catal. 1994, 146, 323–334. [Google Scholar] [CrossRef]
- Chen, K.; Bell, A.T.; Iglesia, E. The Relationship between the Electronic and Redox Properties of Dispersed Metal Oxides and Their Turnover Rates in Oxidative Dehydrogenation Reactions. J. Catal. 2002, 209, 35–42. [Google Scholar] [CrossRef]
- Alvarez-Amparán, M.A.; Cedeño-Caero, L. MoOx-VOx based catalysts for the oxidative desulfurization of refractory compounds: Influence of MoOx-VOx interaction on the catalytic performance. Catal. Today 2017, 282, 133–139. [Google Scholar] [CrossRef]
- Liu, X.; Duan, L.; Yang, W.; Zhu, X. Oxidative dehydrogenation of n-butane to butenes on Mo-doped VMgO catalysts. RSC Adv. 2017, 7, 34131–34137. [Google Scholar] [CrossRef] [Green Version]
- Hussain, O.M.; Srinivasa Rao, K.; Madhuri, K.V.; Ramana, C.V.; Naidu, B.S.; Pai, S.; John, J.; Pinto, R. Growth and characteristics of reactive pulsed laser deposited molybdenum trioxide thin films. Appl. Phys. A 2002, 75, 417–422. [Google Scholar] [CrossRef]
- Fleisch, T.H.; Mains, G.J. An XPS study of the UV reduction and photochromism of MoO3 and WO3. J. Chem. Phys. 1982, 76, 780–786. [Google Scholar] [CrossRef]
- Du, Y.; Li, G.; Peterson, E.W.; Zhou, J.; Zhang, X.; Mu, R.; Dohnálek, Z.; Bowden, M.; Lyubinetsky, I.; Chambers, S.A. Iso-oriented monolayer α-MoO3(010) films epitaxially grown on SrTiO3(001). Nanoscale 2016, 8, 3119–3124. [Google Scholar] [CrossRef] [PubMed]
- Matralis, H.K.; Papadopoulou, C.; Kordulis, C.; Aguilar Elguezabal, A.; Cortes Corberan, V. Selective oxidation of toluene over V2O5/TiO2 catalysts. Effect of vanadium loading and of molybdenum addition on the catalytic properties. Appl. Catal. A 1995, 126, 365–380. [Google Scholar] [CrossRef]
- Bañares, M.A.; Wachs, I.E. Molecular structures of supported metal oxide catalysts under different environments. J. Raman Spectrosc. 2002, 33, 359–380. [Google Scholar] [CrossRef]
- Deo, G.; Wachs, I.E. Predicting molecular structures of surface metal oxide species on oxide supports under ambient conditions. J. Phys. Chem. 1991, 95, 5889–5895. [Google Scholar] [CrossRef]
- Yang, S.; Iglesia, E.; Bell, A.T. Oxidative Dehydrogenation of Propane over V2O5/MoO3/Al2O3 and V2O5/Cr2O3/Al2O3: Structural Characterization and Catalytic Function. J. Phys. Chem. B 2005, 109, 8987–9000. [Google Scholar] [CrossRef] [PubMed]
- Bañares, M.A.; Khatib, S.J. Structure–activity relationships in alumina-supported molybdena–vanadia catalysts for propane oxidative dehydrogenation. Catal. Today 2004, 96, 251–257. [Google Scholar] [CrossRef]
- Rischard, J.; Antinori, C.; Maier, L.; Deutschmann, O. Oxidative dehydrogenation of n-butane to butadiene with Mo-V-MgO catalysts in a two-zone fluidized bed reactor. Appl. Catal. A 2016, 511, 23–30. [Google Scholar] [CrossRef]
- Téllez, C.; Abon, M.; Dalmon, J.A.; Mirodatos, C.; Santamaría, J. Oxidative Dehydrogenation of Butane over VMgO Catalysts. J. Catal. 2000, 195, 113–124. [Google Scholar] [CrossRef]
- Harlin, M.E.; Niemi, V.M.; Krause, A.O.I. Alumina-Supported Vanadium Oxide in the Dehydrogenation of Butanes. J. Catal. 2000, 195, 67–78. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.K.; Hong, U.G.; Yoo, Y.; Cho, Y.-J.; Lee, J.; Jang, H.-S.; Jung, J.C.; Song, I.K. Effect of oxygen capacity and oxygen mobility of supported Mg3(VO4)2 catalysts on the performance in the oxidative dehydrogenation of n-butane. J. Ind. Eng. Chem. 2012, 18, 808–813. [Google Scholar] [CrossRef]
- Guerrero-Pérez, M.O.; Herrera, M.C.; Malpartida, I.; Larrubia, M.A.; Alemany, L.J. Characterization and FT-IR study of nanostructured alumina-supported V-Mo-W-O catalysts. Catal. Today 2006, 118, 360–365. [Google Scholar] [CrossRef]
- Cao, E.; Sankar, M.; Nowicka, E.; He, Q.; Morad, M.; Miedziak, P.J.; Taylor, S.H.; Knight, D.W.; Bethell, D.; Kiely, C.J.; et al. Selective suppression of disproportionation reaction in solvent-less benzyl alcohol oxidation catalysed by supported Au–Pd nanoparticles. Catal. Today 2013, 203, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Kubů, M.; Žilková, N.; Zones, S.I.; Chen, C.-Y.; Al-Khattaf, S.; Čejka, J. Three-dimensional 10-ring zeolites: The activities in toluene alkylation and disproportionation. Catal. Today 2016, 259, 97–106. [Google Scholar] [CrossRef]
- Guisnet, M.; Pinard, L. Characterization of acid-base catalysts through model reactions. Catal. Rev. 2018, 60, 337–436. [Google Scholar] [CrossRef]











| Mo/V Molar Ratio | Tdes Range (°C) | NH3 Desorbed (μmol·g−1) | Weak Acidity (μmol·g−1) | Strong Acidity (μmol·g−1) |
|---|---|---|---|---|
| 0.33 | 280–450 | 7.8 | 4.7 | 3.1 |
| 0.5 | 280–450 | 6.9 | 3.65 | 3.25 |
| 1 | 310–500 | 5.6 | 1.9 | 3.7 |
| 2 | 290–520 | 3.8 | 0.75 | 3.05 |
| Mo/V Molar Ratio | Tdes (°C) | H2 Uptake (mmol·m−2) | Total H2 Uptake (mmol·m−2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LT | HT1 | HT2 | HT3 | LT | HT1 | HT2 | HT3 | ||
| 0.33 | - | 603 | 668 | 700 | - | 0.01 | 0.16 | 0.10 | 0.26 |
| 0.5 | - | 603 | 656 | 700 | - | 0.12 | 0.32 | 1.13 | 1.56 |
| 1 | 403 | 627 | - | 700 | 0.05 | 0.03 | - | 0.25 | 0.33 |
| 2 | 446 | 603 | - | 700 | 0.02 | 0.03 | - | 0.83 | 0.88 |
| Mo/V Molar Ratio | Mo 3d5/2 (BE, eV) | V 2p3/2 (BE, eV) | Mo/V Atomic Ratio (XPS) | ||
|---|---|---|---|---|---|
| C1 Mo5+ | C2 Mo6+(MoO3) | C3 Mo6+(V2MoO8) | |||
| 0.33 | 231.5 | 232.5 | 233.2 | 517.3 | 2 |
| 0.5 | 231.5 | 232.5 | 233.2 | 517.3 | 3 |
| 1 | 231.5 | 232.7 | 233.4 | 517.6 | 5 |
| 2 | 231.5 | 232.6 | 233.2 | 517.3 | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitran, G.; Neaţu, F.; Pavel, O.D.; Trandafir, M.M.; Florea, M. Behavior of Molybdenum–Vanadium Mixed Oxides in Selective Oxidation and Disproportionation of Toluene. Materials 2019, 12, 748. https://doi.org/10.3390/ma12050748
Mitran G, Neaţu F, Pavel OD, Trandafir MM, Florea M. Behavior of Molybdenum–Vanadium Mixed Oxides in Selective Oxidation and Disproportionation of Toluene. Materials. 2019; 12(5):748. https://doi.org/10.3390/ma12050748
Chicago/Turabian StyleMitran, Gheorghita, Florentina Neaţu, Octavian D. Pavel, Mihaela M. Trandafir, and Mihaela Florea. 2019. "Behavior of Molybdenum–Vanadium Mixed Oxides in Selective Oxidation and Disproportionation of Toluene" Materials 12, no. 5: 748. https://doi.org/10.3390/ma12050748