Exploring Tantalum as a Potential Dopant to Promote the Thermoelectric Performance of Zinc Oxide
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Evolution of Structural and Microstructural Properties
3.2. Electronic Transport Properties and Optical Band Gap
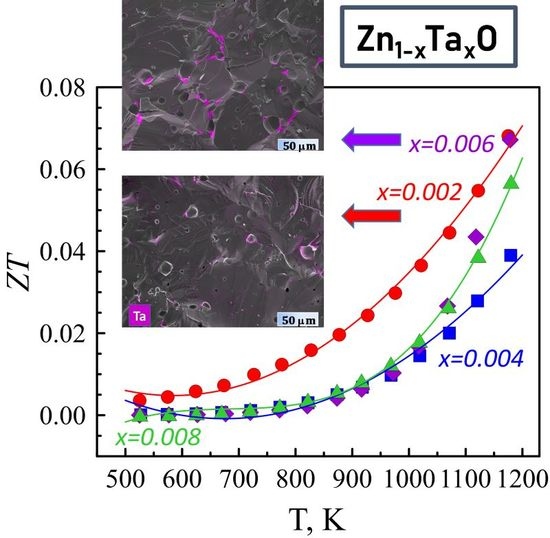
3.3. Thermoelectric Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Vizel, R.; Bargig, T.; Beeri, O.; Gelbstein, Y. Bonding of Bi2Te3-Based Thermoelectric Legs to Metallic Contacts Using Bi0.82Sb0.18 Alloy. J. Electron. Mater. 2016, 45, 1296–1300. [Google Scholar] [CrossRef]
- Hazan, E.; Madar, N.; Parag, M.; Casian, V.; Ben-Yehuda, O.; Gelbstein, Y. Effective Electronic Mechanisms for Optimizing the Thermoelectric Properties of GeTe-Rich Alloys. Adv. Electron. Mater. 2015, 1, 1500228. [Google Scholar] [CrossRef]
- Backhaus-Ricoult, M.; Rustad, J.; Moore, L.; Smith, C.; Brown, J. Semiconducting large bandgap oxides as potential thermoelectric materials for high-temperature power generation? Appl. Phys. A Mater. Sci. Process. 2014, 116, 433–470. [Google Scholar] [CrossRef]
- Morkoç, H.; Özgür, Ü. Zinc Oxide: Fundamentals, Materials and Device Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; ISBN 9783527408139. [Google Scholar]
- Tsubota, T.; Ohtaki, M.; Eguchi, K.; Arai, H. Thermoelectric properties of Al-doped ZnO as a promising oxide material for high-temperature thermoelectric conversion. J. Mater. Chem. 1997, 7, 85–90. [Google Scholar] [CrossRef]
- Ohtaki, M.; Tsubota, T.; Eguchi, K.; Arai, H. High-temperature thermoelectric properties of (Zn1−xAlx)O. J. Appl. Phys. 1996, 79, 1816–1818. [Google Scholar] [CrossRef]
- Bérardan, D.; Byl, C.; Dragoe, N. Influence of the preparation conditions on the thermoelectric properties of Al-doped ZnO. J. Am. Ceram. Soc. 2010, 93, 2352–2358. [Google Scholar] [CrossRef]
- Jood, P.; Mehta, R.J.; Zhang, Y.; Borca-Tasciuc, T.; Dou, S.X.; Singh, D.J.; Ramanath, G. Heavy element doping for enhancing thermoelectric properties of nanostructured zinc oxide. RSC Adv. 2014, 4, 6363. [Google Scholar] [CrossRef]
- Hopper, E.M.; Zhu, Q.; Song, J.H.; Peng, H.; Freeman, A.J.; Mason, T.O. Electronic and thermoelectric analysis of phases in the In2O3(ZnO)k system. J. Appl. Phys. 2011, 109, 013713. [Google Scholar] [CrossRef]
- Liang, X. Thermoelectric transport properties of Fe-enriched ZNO with high-temperature nanostructure refinement. ACS Appl. Mater. Interfaces 2015, 7, 7927–7937. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Choi, J.W.; Kim, S.J.; Kim, G.H.; Cho, Y.S. Zn1−xBixO (0 ≤ x ≤ 0.02) for thermoelectric power generations. J. Alloys Compd. 2009, 485, 532–537. [Google Scholar] [CrossRef]
- Ohtaki, M.; Araki, K.; Yamamoto, K. High thermoelectric performance of dually doped ZnO ceramics. J. Electron. Mater. 2009, 38, 1234–1238. [Google Scholar] [CrossRef]
- Zhang, D.B.; Zhang, B.P.; Ye, D.S.; Liu, Y.C.; Li, S. Enhanced Al/Ni co-doping and power factor in textured ZnO thermoelectric ceramics prepared by hydrothermal synthesis and spark plasma sintering. J. Alloys Compd. 2016, 656, 784–792. [Google Scholar] [CrossRef]
- Park, K.; Ko, K.Y.; Seo, W.-S.; Cho, W.-S.; Kim, J.-G.; Kim, J.Y. High-temperature thermoelectric properties of polycrystalline Zn1−x−yAlxTiyO ceramics. J. Eur. Ceram. Soc. 2006, 27, 813–817. [Google Scholar] [CrossRef]
- Zakharchuk, K.V.; Tobaldi, D.M.; Xiao, X.; Xie, W.; Mikhalev, S.M.; Martins, J.F.; Frade, J.R.; Weidenkaff, A.; Kovalevsky, A.V. Synergistic effects of zirconium- and aluminum co-doping on the thermoelectric performance of zinc oxide. J. Eur. Ceram. Soc. 2019, 39, 1222–1229. [Google Scholar] [CrossRef]
- Zakharchuk, K.V.; Widenmeyer, M.; Alikin, D.O.; Xie, W.; Populoh, S.; Mikhalev, S.M.; Tselev, A.; Frade, J.R.; Weidenkaff, A.; Kovalevsky, A.V. A self-forming nanocomposite concept for ZnO-based thermoelectrics. J. Mater. Chem. A 2018, 6, 13386–13396. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.N.; Xin, H.X.; Li, D.; Li, X.J.; Zhang, J.; Qin, X.Y. High temperature thermoelectric properties of Nb-doped ZnO ceramics. J. Phys. Chem. Solids 2013, 74, 1811–1815. [Google Scholar] [CrossRef]
- Kong, J.Z.; Li, A.D.; Zhai, H.F.; Gong, Y.P.; Li, H.; Wu, D. Preparation, characterization of the Ta-doped ZnO nanoparticles and their photocatalytic activity under visible-light illumination. J. Solid State Chem. 2009, 182, 2061–2067. [Google Scholar] [CrossRef]
- Herrera, V.; Díaz-Becerril, T.; Reyes-Cervantes, E.; García-Salgado, G.; Galeazzi, R.; Morales, C.; Rosendo, E.; Coyopol, A.; Romano, R.; Nieto-Caballero, F. Highly Visible Photoluminescence from Ta-Doped Structures of ZnO Films Grown by HFCVD. Crystals 2018, 8, 395. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Li, M.; Li, H.; Xu, S.; Wu, X.; Yang, B. Microstructural and optical properties of Ta-doped ZnO films prepared by radio frequency magnetron sputtering. Ceram. Int. 2016, 42, 10847–10853. [Google Scholar] [CrossRef]
- Richard, D.; Romero, M.; Faccio, R. Experimental and theoretical study on the structural, electrical and optical properties of tantalum-doped ZnO nanoparticles prepared via sol-gel acetate route. Ceram. Int. 2018, 44, 703–711. [Google Scholar] [CrossRef]
- Gao, Z.; Myung, Y.; Huang, X.; Kanjolia, R.; Park, J.; Mishra, R.; Banerjee, P. Doping Mechanism in Transparent, Conducting Tantalum Doped ZnO Films Deposited Using Atomic Layer Deposition. Adv. Mater. Interfaces 2016, 3, 1600496. [Google Scholar] [CrossRef]
- Mahmood, K.; Song, D.; Park, S. Bin Effects of thermal treatment on the characteristics of boron and tantalum-doped ZnO thin films deposited by the electrospraying method at atmospheric pressure. Surf. Coatings Technol. 2012, 206, 4730–4740. [Google Scholar] [CrossRef]
- Kovalevsky, A.V.V.; Yaremchenko, A.A.A.; Populoh, S.; Weidenkaff, A.; Frade, J.R.R. Enhancement of thermoelectric performance in strontium titanate by praseodymium substitution. J. Appl. Phys. 2013, 113, 053704. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter. 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Soumahoro, I.; Colis, S.; Schmerber, G.; Leuvrey, C.; Barre, S.; Ulhaq-Bouillet, C.; Muller, D.; Abd-lefdil, M.; Hassanain, N.; Petersen, J.; et al. Structural, optical, spectroscopic and electrical properties of Mo-doped ZnO thin films grown by radio frequency magnetron sputtering. Thin Solid Films 2014, 566, 61–69. [Google Scholar] [CrossRef]
- Ferrari, C.R.; Hernandes, A.C. MgTa2O6 and ZnTa2O6 ceramics from oxide precursors. J. Eur. Ceram. Soc. 2002, 22, 2101–2105. [Google Scholar] [CrossRef]
- Colak, H. Synthesis and characterization of CeO2-doped ZnO. Met. Mater. 2016, 54, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Godavarti, U.; Mote, V.D.; Dasari, M. Role of cobalt doping on the electrical conductivity of ZnO nanoparticles. J. Asian Ceram. Soc. 2017, 5, 391–396. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Wang, X.; Fu, B.J.; Liu, Y.H.; Ding, Y.Z.; Yue, Z.X. Structure and properties of low temperature sintered ZnTa 2O 6 microwave dielectric ceramics. Ceram. Int. 2012, 38, S169–S172. [Google Scholar] [CrossRef]
- Dolgonos, A.; Mason, T.O.; Poeppelmeier, K.R. Direct optical band gap measurement in polycrystalline semiconductors: A critical look at the Tauc method. J. Solid State Chem. 2016, 240, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Sernelius, B.E.; Berggren, K.F.; Jin, Z.C.; Hamberg, I.; Granqvist, C.G. Band-gap tailoring of ZnO by means of heavy Al doping. Phys. Rev. B 1988, 37, 10244–10248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Sagar, P.; Mehra, R.M. Band gap widening and narrowing in moderately and heavily doped n-ZnO films. Solid. State. Electron. 2006, 50, 1420–1424. [Google Scholar] [CrossRef]
- Ohno, Y.; Koizumi, H.; Taishi, T.; Yonenaga, I.; Fujii, K.; Goto, H.; Yao, T. Optical properties of dislocations in wurtzite ZnO single crystals introduced at elevated temperatures. J. Appl. Phys. 2008, 104, 073515. [Google Scholar] [CrossRef]
- Weiss, D.S.; Abkowitz, M.; Kasap, S.; Capper, P. Handbook of Electronic and Photonic Materials; Kasap, S., Capper, P., Eds.; Springer International Publishing: Cham, Switzerland, 2006; Volume 10, ISBN 978-3-319-48931-5. [Google Scholar]
- Takemoto, H.; Fugane, K.; Yan, P.; Drennan, J.; Saito, M.; Mori, T.; Yamamura, H. Reduction of thermal conductivity in dually doped ZnO by design of three-dimensional stacking faults. RSC Adv. 2014, 4, 2661–2672. [Google Scholar] [CrossRef]
- Kim, K.H.; Shim, S.H.; Shim, K.B.; Niihara, K.; Hojo, J. Microstructural and thermoelectric characteristics of zinc oxide-based thermoelectric materials fabricated using a spark plasma sintering process. J. Am. Ceram. Soc. 2005, 88, 628–632. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Chonan, Y.; Oda, M.; Komiyama, T.; Aoyama, T.; Sugiyama, S. Thermoelectric properties of ZnO ceramics Co-doped with Al and transition metals. J. Electron. Mater. 2011, 40, 723–727. [Google Scholar] [CrossRef]








| x | ρexp/ρtheor * (%) | Activation Energy of the Electronic Transport | Eg ** (eV) | |
|---|---|---|---|---|
| Temperature Range (K) | Ea (kJ/mol) | |||
| 0 | 91 | 873–1177 675–873 | 54 ± 2 48 ± 4 | 3.14 [17] |
| 0.02 | 92 | - | - | 3.24 |
| 0.04 | 93 | 822–1180 573–822 | 18 ± 1 23 ± 1 | 3.21 |
| 0.06 | 93 | 820–1179 525–820 | 22 ± 1 23 ± 1 | 3.21 |
| 0.08 | 94 | 822–1180 522–822 | 22 ± 1 12 ± 2 | 3.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Serrano, B.I.; Xie, W.; Aguirre, M.H.; Tobaldi, D.M.; Sarabando, A.R.; Rasekh, S.; Mikhalev, S.M.; Frade, J.R.; Weidenkaff, A.; Kovalevsky, A.V. Exploring Tantalum as a Potential Dopant to Promote the Thermoelectric Performance of Zinc Oxide. Materials 2019, 12, 2057. https://doi.org/10.3390/ma12132057
Arias-Serrano BI, Xie W, Aguirre MH, Tobaldi DM, Sarabando AR, Rasekh S, Mikhalev SM, Frade JR, Weidenkaff A, Kovalevsky AV. Exploring Tantalum as a Potential Dopant to Promote the Thermoelectric Performance of Zinc Oxide. Materials. 2019; 12(13):2057. https://doi.org/10.3390/ma12132057
Chicago/Turabian StyleArias-Serrano, Blanca I., Wenjie Xie, Myriam H. Aguirre, David M. Tobaldi, Artur R. Sarabando, Shahed Rasekh, Sergey M. Mikhalev, Jorge R. Frade, Anke Weidenkaff, and Andrei V. Kovalevsky. 2019. "Exploring Tantalum as a Potential Dopant to Promote the Thermoelectric Performance of Zinc Oxide" Materials 12, no. 13: 2057. https://doi.org/10.3390/ma12132057