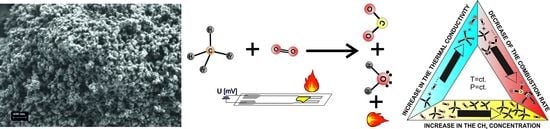
CeO2:Mn3O4 Catalytic Micro-Converters Tuned for CH4 Detection Based on Catalytic Combustion under Real Operating Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials’ Preparation
2.2. Materials’ Deposition and Temperature Calibration
2.3. Materials’ Characterization
2.4. Electrical Characterization
3. Results
3.1. Materials’ Characterization
3.1.1. Raman Spectroscopy
3.1.2. X-ray Diffraction Analysis
3.1.3. X-ray Photoelectron Spectroscopy
3.1.4. Temperature Programmed Reduction Analysis
3.1.5. Textural Characterization
3.1.6. Scanning Electron Microscopy
3.2. CH4 Catalytic Combustion Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biro, A.; Pap, A.; Barsony, I.; Ducso, C.S. Micro-pellistor with integrated porous alumina catalyst support. Procedia Eng. 2014, 87, 200–203. [Google Scholar] [CrossRef] [Green Version]
- Krebs, P.; Grisel, A. A low power integrated catalytic gas sensor. Sens. Actuators B: Chem. 1993, 13, 155–158. [Google Scholar] [CrossRef]
- Aigner, R.; Auerbach, F.; Huber, P.; Mueller, R.; Scheller, G. Sinusoidal temperature modulation of the Si-Planar-Pellistor. Sens. Actuators B Chem. 1994, 18–19, 143–147. [Google Scholar] [CrossRef]
- Li, T.; Xu, L.; Wang, Y. Micro-Heater Based Gase Sensors; Micro Electro Mechanical Systems; Springer: Singapore, 2018; pp. 1–37. [Google Scholar]
- Nemirovsky, Y.; Stolyarova, S.; Blank, T.; Bar-Lev, S.; Svetlitza, A.; Zviagintsev, A.; Brouk, I. A new pellistor-like gas sensor based on micromachined CMOS transistor. IEEE Trans. Electron Devices 2018, 65, 5494–5498. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, T.; Wang, H.; Tang, C.; Zhang, L. A Novel Fabricating Process of Catalytic Gas Sensor Based on Droplet Generating Technology. Micromachines 2019, 10, 71. [Google Scholar] [CrossRef] [Green Version]
- Gardner, T.; Manginelli, R.; Lewis, P.; Frye-Mason, G.C.; Colburn, C. U.S. Patent 6,786,716 B1, 7 September 2004.
- Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials. Catal. Rev. Sci. Eng. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Liang, Q.; Wu, X.; Weng, D.; Xu, H. Oxygen activation on Cu/Mn–Ce mixed oxides and the role in diesel soot oxidation. Catal. Today 2008, 139, 113–118. [Google Scholar] [CrossRef]
- Spassova, I.; Tsontcheva, T.; Velichkova, N.; Khristova, M.; Nihtianova, D. Catalytic reduction of NO with decomposed methanol on alumina-supported Mn–Ce catalysts. J. Colloid Interface Sci. 2012, 374, 267–277. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, Q. Recent progress in synergistic catalysis over heterometallic nanoparticles. J. Mater. Chem. 2011, 21, 13705–13725. [Google Scholar] [CrossRef]
- Neatu, S.; Trandafir, M.M.; Stanoiu, A.; Florea, O.G.; Simion, C.E.; Leonat, L.N.; Cobianu, C.; Gheorghe, M.; Florea, M.; Neatu, F. Bulk versus surface modification of alumina with Mn and Ce based oxides for CH4 catalytic combustion. Materials 2019, 12, 1771. [Google Scholar] [CrossRef] [Green Version]
- Venkataswamy, P.; Jampaiah, D.; Lin, F.; Alxneit, I.; Reddy, B.M. Structural properties of alumina supported Ce-Mn solid solutions and their markedly enhanced catalytic activity for CO oxidation. Appl. Surf. Sci. 2015, 349, 299–309. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Investigations into nanostructured ceria–zirconia catalysts for soot combustion. Appl. Catal. B: Environ. 2016, 180, 271–282. [Google Scholar] [CrossRef]
- An, K.; Somorjai, G.A. Nanocatalysis I: Synthesis of metal and bimetallic nanoparticles and porous oxides and their catalytic reaction studies. Catal. Lett. 2015, 145, 233–248. [Google Scholar] [CrossRef]
- Védrine, J.C. Revisiting active sites in heterogeneous catalysis: Their structure and their dynamic behavior. Appl. Catal. A 2014, 474, 40–50. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; He, H.; Wu, Z.; Wu, J.; Chen, L.; Ye, D.; Fu, M. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Appl. Catal. B 2018, 223, 91–102. [Google Scholar] [CrossRef]
- Wu, X.; Liu, S.; Weng, D.; Lin, F.; Ran, R. MnOx–CeO2–Al2O3 mixed oxides for soot oxidation: Activity and thermal stability. J. Hazard. Mater. 2011, 187, 283–290. [Google Scholar] [CrossRef]
- Sato, T.; Komanoya, T. Selective oxidation of alcohols with molecular oxygen catalyzed by Ru/MnOx/CeO2 under mild conditions. Catal. Commun. 2009, 10, 1095–1098. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Rao, K.N.; Jampaiah, D.; Reddy, B.M. Nanostructured manganese doped ceria solid solutions for CO oxidation at lower temperatures. Appl. Catal. B 2015, 162, 122–132. [Google Scholar] [CrossRef]
- Fazio, B.; Spadaro, L.; Trunfio, G.; Negro, J.; Arena, F. Raman scattering of MnOx-CeOx composite catalysts: Structural aspects and laser-heating effects. J. Raman Spectrosc. 2011, 42, 1583–1588. [Google Scholar] [CrossRef]
- He, H.; Lin, X.; Li, S.; Wu, Z.; Gao, J.; W, J.; Wen, W.; Ye, D.; Fu, M. The key surface species and oxygen vacancies in MnOx(0.4)-CeO2 toward repeated soot oxidation. Appl. Catal. B: Environ. 2018, 223, 134–142. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Chen, Y.-C.; Lin, Y.-G.; Chen, L.-C.; Chen, K.-H. Reversible phase transformation of MnO2 nanosheets in an electrochemical capacitor investigated by in situ Raman spectroscopy. Chem. Commun. 2011, 47, 1252–1254. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, Y.; Huang, X.; Xu, Y.; Zhu, H.; Wang, J.; Shen, W. MnOx–CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcination temperature. Appl. Catal. B: Environ. 2006, 62, 265–273. [Google Scholar] [CrossRef]
- Machida, M.; Uto, M.; Kurogi, D.; Kijima, T. MnOx-CeO2 Binary oxides for catalytic NOx sorption at low temperatures. sorptive removal of NOx. Chem. Mater. 2000, 12, 3158–3164. [Google Scholar] [CrossRef]
- Santos, V.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl. Catal. B: Environ. 2010, 99, 353–363. [Google Scholar] [CrossRef]
- Moses Ezhil Raj, A.; Grace Victoria, S.; Bena Jothy, V.; Ravidhas, C.; Wollschlager, J.; Suendorf, M.; Neumann, M.; Jayanchandran, M.; Sanjeeviraja, C. XRD and XPS characterization of mixed valence Mn3O4 hausmannite thin films prepared by chemical spray pyrolysis technique. Appl. Surf Sci. 2010, 256, 2920–2926. [Google Scholar] [CrossRef]
- Tholkappiyan, R.; Naveen, A.N.; Vishista, K.; Hamed, F. Investigation on the electrochemical performance of hausmannite Mn3O4 nanoparticles by ultrasonic irradiation assisted co-precipitation method for supercapacitor electrodes. J. Taibah Univ. Sci. 2018, 12, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Geng, Z.; Wang, Y.; Liu, J.; Li, G.; Li, L.; Huang, K.; Yuan, L.; Feng, S. δ-MnO2–Mn3O4 nanocomposite for photochemical water oxidation: Active structure stabilized in the interface. ACS Appl. Mater. Interfaces 2016, 8, 27825–27831. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Muroyama, H.; Hano, S.; Matsui, T.; Eguchi, K. Catalytic soot combustion over CeO2-based oxides. Catal. Today 2010, 153, 133–135. [Google Scholar] [CrossRef]
- Li, H.; Qi, G.; Tana, T.; Zhang, X.; Li, W.; Shen, W. Morphological impact of manganese–cerium oxides on ethanol oxidation. Catal. Sci. Technol. 2011, 1, 1677–1682. [Google Scholar] [CrossRef]
- Ahlers, S.; Mueller, G.; Becker, T.; Doll, T. Factors influencing the gas sensitivity of metal oxide Materials. Encycl. Sens. 2006, 3, 413–447. [Google Scholar]
- Nagy, E. Membrane Gas Separation. In Basic Equations of Mass Transport through a Membrane Layer; Elsevier: Amsterdam, The Netherlands, 2019; pp. 457–481. Available online: https://doi.org/10.1016/C2016-0-04043-3 (accessed on 8 May 2020).
- Lee, J.H.; Trimm, D.L. Catalytic combustion of methane. Fuel Process. Technol. 1995, 42, 339–359. [Google Scholar] [CrossRef]
- Chou, J. Hazardous Gas Monitors, A Practical Guide to Selection, Operation and Applications; McGraw-Hill Book Company: New York, NY, USA, 2000. [Google Scholar]
- Prasad, R.C.; Mani, N.; Venart, J.E.S. Thermal conductivity of methane. Int. J. Thermophys. 1984, 5, 265–279. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglieri, G. Solid State Gas Sensing. Springer: Berlin, Germany, 2009; p. 242. [Google Scholar]
- Kolve, S.D.; Adam, M.; Ducso, C.; Barsony, I.; Cobianu, C.; Van Den Berg, A. Thermal modelling of a porous silicon-based pellistor-type catalytic flammable gas sensor with two supporting beams. Microelectron. J. 2000, 31, 339–342. [Google Scholar] [CrossRef]















| Sample | Composition | Ce/Mn Molar Ratio | Lattice Parameters | Crystallite Size (nm) | |
|---|---|---|---|---|---|
| (Ce/Mn) a | (Ce/Mn) b | a0 (nm) | |||
| CeO2:Mn3O4 (7:3) | CeO2 | 2.33 | 0.23 | 0.536 | 6 |
| Mn3O4 | 27 | ||||
| CeO2:Mn3O4 (3:7) | CeO2 | 0.43 | 0.11 | 0.533 | 7 |
| Mn3O4 | 33 | ||||
| MnO2 | 10 | ||||
| Sample | BET Surface Area (m2⋅g−1) | Pore Volume (cm3⋅g−1) | Pore Size (nm) | Average Grains Size (nm) (a) |
|---|---|---|---|---|
| CeO2:Mn3O4 (7:3) | 52 | 0.35 | 29 | 16 |
| CeO2:Mn3O4 (3:7) | 29 | 0.30 | 43 | 29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simion, C.E.; Florea, O.G.; Florea, M.; Neaţu, F.; Neaţu, Ş.; Trandafir, M.M.; Stănoiu, A. CeO2:Mn3O4 Catalytic Micro-Converters Tuned for CH4 Detection Based on Catalytic Combustion under Real Operating Conditions. Materials 2020, 13, 2196. https://doi.org/10.3390/ma13092196
Simion CE, Florea OG, Florea M, Neaţu F, Neaţu Ş, Trandafir MM, Stănoiu A. CeO2:Mn3O4 Catalytic Micro-Converters Tuned for CH4 Detection Based on Catalytic Combustion under Real Operating Conditions. Materials. 2020; 13(9):2196. https://doi.org/10.3390/ma13092196
Chicago/Turabian StyleSimion, Cristian E., Ovidiu G. Florea, Mihaela Florea, Florentina Neaţu, Ştefan Neaţu, Mihaela M. Trandafir, and Adelina Stănoiu. 2020. "CeO2:Mn3O4 Catalytic Micro-Converters Tuned for CH4 Detection Based on Catalytic Combustion under Real Operating Conditions" Materials 13, no. 9: 2196. https://doi.org/10.3390/ma13092196