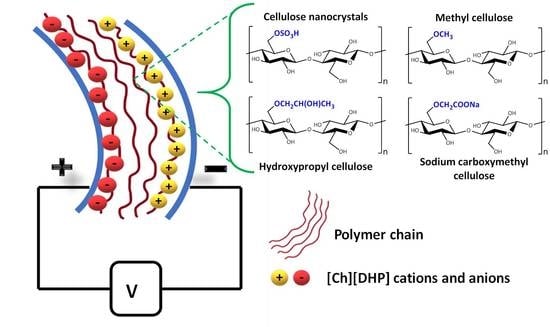
Cellulose Nanocrystal and Water-Soluble Cellulose Derivative Based Electromechanical Bending Actuators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
3.1. Morphological Characterization
3.2. Molecular and Crystalline Structure
3.3. Mechanical Properties
3.4. Electrical Properties
3.5. Bending Actuation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Correia, D.M.; Fernandes, L.C.; Martins, P.M.; García-Astrain, C.; Costa, C.M.; Reguera, J.; Lanceros-Mendez, S. Ionic Liquid-Polymer Composites: A New Platform for Multifunctional Applications. Adv. Funct. Mater. 2020, 1909736. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatraman, V.; Evjen, S.; Knuutila, H.K.; Fiksdahl, A.; Alsberg, B.K. Predicting ionic liquid melting points using machine learning. J. Mol. Liq. 2018, 264, 318–326. [Google Scholar] [CrossRef]
- Mejri, R.; Dias, J.C.; Hentati, S.B.; Martins, M.S.; Costa, C.M.; Lanceros-Mendez, S. Effect of anion type in the performance of ionic liquid/poly(vinylidene fluoride) electromechanical actuators. J. Non Cryst. Solids 2016, 453, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, S.; Quartarone, E.; Mustarelli, P.; Magistris, A.; Fagnoni, M.; Protti, S.; Gerbaldi, C.; Spinella, A. Lithium ion conducting PVdF-HFP composite gel electrolytes based on N-methoxyethyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)-imide ionic liquid. J. Power Sour. 2010, 195, 559–566. [Google Scholar] [CrossRef]
- Correia, D.M.; Costa, C.M.; Lizundia, E.; Sabater i Serra, R.; Gómez-Tejedor, J.A.; Biosca, L.T.; Meseguer-Dueñas, J.M.; Gomez Ribelles, J.L.; Lanceros-Méndez, S. Influence of cation and anion type on the formation of the electroactive β-phase and thermal and dynamic mechanical properties of poly(vinylidene fluoride)/ionic liquids blends. J. Phys. Chem. C 2019, 123, 27917–27926. [Google Scholar] [CrossRef]
- Liu, Y.; Ghaffari, M.; Zhao, R.; Lin, J.-H.; Lin, M.; Zhang, Q.M. Enhanced electromechanical response of ionic polymer actuators by improving mechanical coupling between ions and polymer matrix. Macromolecules 2012, 45, 5128–5133. [Google Scholar] [CrossRef]
- Bennett, M.D.; Leo, D.J. Ionic liquids as stable solvents for ionic polymer transducers. Sens. Actuators A Phys. 2004, 115, 79–90. [Google Scholar] [CrossRef]
- Xing, C.; Zhao, M.; Zhao, L.; You, J.; Cao, X.; Li, Y. Ionic liquid modified poly(vinylidene fluoride): Crystalline structures, miscibility, and physical properties. Polym. Chem. 2013, 4, 5726–5734. [Google Scholar] [CrossRef]
- Dias, J.C.; Correia, D.M.; Costa, C.M.; Ribeiro, C.; Maceiras, A.; Vilas, J.L.; Botelho, G.; de Zea Bermudez, V.; Lanceros-Mendez, S. Improved response of ionic liquid-based bending actuators by tailored interaction with the polar fluorinated polymer matrix. Electrochim. Acta 2019, 296, 598–607. [Google Scholar] [CrossRef]
- Mejri, R.; Dias, J.C.; Besbes Hentati, S.; Botelho, G.; Esperança, J.M.S.S.; Costa, C.M.; Lanceros-Mendez, S. Imidazolium-based ionic liquid type dependence of the bending response of polymer actuators. Eur. Polym. J. 2016, 85, 445–451. [Google Scholar] [CrossRef]
- Correia, D.M.; Barbosa, J.C.; Costa, C.M.; Reis, P.M.; Esperança, J.M.S.S.; de Zea Bermudez, V.; Lanceros-Méndez, S. Ionic liquid cation size-dependent electromechanical response of ionic liquid/poly(vinylidene fluoride)-based soft actuators. J. Phys. Chem. C 2019, 123, 12744–12752. [Google Scholar] [CrossRef]
- Xu, P.; Cui, Z.-P.; Ruan, G.; Ding, Y.-S. Enhanced crystallization kinetics of PLLA by ethoxycarbonyl ionic liquid modified graphene. Chin. J. Polym. Sci. 2019, 37, 243–252. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, Z.; Yin, G.; Chen, G.-X.; Li, Q. Effect of ionic liquid-containing poly(ε-caprolactone) on the dispersion and dielectric properties of polymer/carbon nanotube composites. RSC Adv. 2016, 6, 31351–31358. [Google Scholar] [CrossRef]
- Elamin, K.; Shojaatalhosseini, M.; Danyliv, O.; Martinelli, A.; Swenson, J. Conduction mechanism in polymeric membranes based on PEO or PVdF-HFP and containing a piperidinium ionic liquid. Electrochim. Acta 2019, 299, 979–986. [Google Scholar] [CrossRef]
- Glatz, H.; Lizundia, E.; Pacifico, F.; Kundu, D. An organic cathode based dual-ion aqueous zinc battery enabled by a cellulose membrane. ACS Appl. Energy Mater. 2019, 2, 1288–1294. [Google Scholar] [CrossRef]
- Kose, O.; Tran, A.; Lewis, L.; Hamad, W.Y.; MacLachlan, M.J. Unwinding a spiral of cellulose nanocrystals for stimuli-responsive stretchable optics. Nat. Commun. 2019, 10, 510. [Google Scholar] [CrossRef]
- Tyagi, P.; Mathew, R.; Opperman, C.; Jameel, H.; Gonzalez, R.; Lucia, L.; Hubbe, M.; Pal, L. High-strength antibacterial chitosan–cellulose nanocrystal composite tissue paper. Langmuir 2019, 35, 104–112. [Google Scholar] [CrossRef]
- Lizundia, E.; Jimenez, M.; Altorfer, C.; Niederberger, M.; Caseri, W. Electroless plating of platinum nanoparticles onto mesoporous cellulose films for catalytically active free-standing materials. Cellulose 2019, 26, 5513–5527. [Google Scholar] [CrossRef]
- Lucchini, M.; Lizundia, E.; Moser, S.; Niederberger, M.; Nystrom, G. Titania-cellulose hybrid monolith for in-flow purification of water under solar illumination. ACS Appl. Mater. Interfaces 2018, 10, 29599–29607. [Google Scholar] [CrossRef]
- Osorio, D.A.; Lee, B.E.J.; Kwiecien, J.M.; Wang, X.; Shahid, I.; Hurley, A.L.; Cranston, E.D.; Grandfield, K. Cross-linked cellulose nanocrystal aerogels as viable bone tissue scaffolds. Acta Biomater. 2019, 87, 152–165. [Google Scholar] [CrossRef]
- Khan, M.K.; Hamad, W.Y.; MacLachlan, M.J. Tunable mesoporous bilayer photonic resins with chiral nematic structures and actuator properties. Adv. Mater. 2014, 26, 2323–2328. [Google Scholar] [CrossRef]
- Nevstrueva, D.; Murashko, K.; Vunder, V.; Aabloo, A.; Pihlajamäki, A.; Mänttäri, M.; Pyrhönen, J.; Koiranen, T.; Torop, J. Natural cellulose ionogels for soft artificial muscles. Colloids Surf. B Biointerfaces 2018, 161, 244–251. [Google Scholar] [CrossRef]
- Lizundia, E.; Puglia, D.; Nguyen, T.-D.; Armentano, I. Cellulose nanocrystal based multifunctional nanohybrids. Prog. Mater. Sci. 2020, 112, 100668. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Li, J.; Ye, S.; Wei, J.; Guo, J. A bio-inspired cellulose nanocrystal-based nanocomposite photonic film with hyper-reflection and humidity-responsive actuator properties. J. Mater. Chem. C 2016, 4, 9687–9696. [Google Scholar] [CrossRef]
- Donhowe, I.G.; Fennema, O. The effects of plasticizers on crystallinity, permeability, and mechanical properties of methylcellulose films. J. Food Process. Preserv. 1993, 17, 247–257. [Google Scholar] [CrossRef]
- Fernandes, L.C.; Correia, D.M.; Pereira, N.; Tubio, C.R.; Lanceros-Méndez, S. Highly sensitive humidity sensor based on ionic liquid–polymer composites. ACS Appl. Polym. Mater. 2019, 1, 2723–2730. [Google Scholar] [CrossRef]
- Dias, J.C.; Martins, M.S.; Ribeiro, S.; Silva, M.M.; Esperança, J.M.S.S.; Ribeiro, C.; Botelho, G.; Costa, C.M.; Lanceros-Mendez, S. Electromechanical actuators based on poly(vinylidene fluoride) with [N1 1 1 2(OH)][NTf2] and [C2mim] [C2SO4]. J. Mater. Sci. 2016, 51, 9490–9503. [Google Scholar] [CrossRef]
- Must, I.; Vunder, V.; Kaasik, F.; Põldsalu, I.; Johanson, U.; Punning, A.; Aabloo, A. Ionic liquid-based actuators working in air: The effect of ambient humidity. Sens. Actuators B Chem. 2014, 202, 114–122. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Sierra, E.; Eguiraun, H.; Lizundia, E. Iridescent cellulose nanocrystal films: The link between structural colour and Bragg’s law. Eur. J. Phys. 2018, 39, 45803. [Google Scholar] [CrossRef] [Green Version]
- Lizundia, E.; Nguyen, T.D.; Vilas, J.L.; Hamad, W.Y.; MacLachlan, M.J. Chiroptical luminescent nanostructured cellulose films. Mater. Chem. Front. 2017, 1, 979–987. [Google Scholar] [CrossRef]
- Lizundia, E.; Nguyen, T.-D.; Vilas, J.L.; Hamad, W.Y.; Maclachlan, M.J. Chiroptical, morphological and conducting properties of chiral nematic mesoporous cellulose/polypyrrole composite films. J. Mater. Chem. A 2017, 5, 19184–19194. [Google Scholar] [CrossRef]
- Liu, P.; Guo, X.; Nan, F.; Duan, Y.; Zhang, J. Modifying mechanical, optical properties and thermal processability of iridescent cellulose nanocrystal films using ionic liquid. ACS Appl. Mater. Interfaces 2017, 9, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.A.; Cortez, A.R.; Barsan, M.M.; Santos, J.B.; Brett, C.M.A.; de Sousa, H.C. Development of greener multi-responsive chitosan biomaterials doped with biocompatible ammonium ionic liquids. ACS Sustain. Chem. Eng. 2013, 1, 1480–1492. [Google Scholar] [CrossRef]
- Lizundia, E.; Goikuria, U.; Vilas, J.L.; Cristofaro, F.; Bruni, G.; Fortunati, E.; Armentano, I.; Visai, L.; Torre, L. Metal nanoparticles embedded in cellulose nanocrystal based films: Material properties and post-use analysis. Biomacromolecules 2018, 19, 2618–2628. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.D.; Guirguis, O.W. Macrostructure and optical studies of hydroxypropyl cellulose in pure and Nano-composites forms. Results Phys. 2019, 15, 102637. [Google Scholar] [CrossRef]
- Tunç, S.; Duman, O.; Polat, T.G. Effects of montmorillonite on properties of methyl cellulose/carvacrol based active antimicrobial nanocomposites. Carbohydr. Polym. 2016, 150, 259–268. [Google Scholar] [CrossRef]
- Luna-Martínez, J.F.; Hernández-Uresti, D.B.; Reyes-Melo, M.E.; Guerrero-Salazar, C.A.; González-González, V.A.; Sepúlveda-Guzmán, S. Synthesis and optical characterization of ZnS–sodium carboxymethyl cellulose nanocomposite films. Carbohydr. Polym. 2011, 84, 566–570. [Google Scholar] [CrossRef]
- Meira, R.M.; Correia, D.M.; Ribeiro, S.; Costa, P.; Gomes, A.C.; Gama, F.M.; Lanceros-Méndez, S.; Ribeiro, C. Ionic-Liquid-Based Electroactive Polymer Composites for Muscle Tissue Engineering. ACS Appl. Polym. Mater. 2019, 1, 2649–2658. [Google Scholar] [CrossRef] [Green Version]
- Lin, N.; Bruzzese, C.; Dufresne, A. TEMPO-oxidized nanocellulose participating as crosslinking aid for alginate-based sponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef]
- Barbosa, P.; Campos, J.; Turygin, A.; Shur, V.Y.; Kholkin, A.; Barros-Timmons, A.; Figueiredo, F.M. Piezoelectric poly(lactide) stereocomplexes with a cholinium organic ionic plastic crystal. J. Mater. Chem. C 2017, 5, 12134–12142. [Google Scholar] [CrossRef] [Green Version]
- Kiyose, M.; Yamamoto, E.; Yamane, C.; Midorikawa, T.; Takahashi, T. Structure and properties of low-substituted hydroxypropylcellulose films and fibers regenerated from aqueous sodium hydroxide solution. Polym. J. 2007, 39, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Gulati, I.; Park, J.; Maken, S.; Lee, M.-G. Production of carboxymethylcellulose fibers from waste lignocellulosic sawdust using NaOH/NaClO2 pretreatment. Fibers Polym. 2014, 15, 680–686. [Google Scholar] [CrossRef]
- Torres-Huerta, A.M.; Del Angel-López, D.; Domínguez-Crespo, M.A.; Palma-Ramírez, D.; Perales-Castro, M.E.; Flores-Vela, A. Morphological and mechanical properties dependence of PLA amount in PET matrix processed by single-screw extrusion. Polym. Plast. Technol. Eng. 2016, 55, 672–683. [Google Scholar] [CrossRef]
- Sun, S.; Mitchell, J.R.; MacNaughtan, W.; Foster, T.J.; Harabagiu, V.; Song, Y.; Zheng, Q. Comparison of the mechanical properties of cellulose and starch films. Biomacromolecules 2010, 11, 126–132. [Google Scholar] [CrossRef] [PubMed]









| Sample | E (MPa) | σy (MPa) | εb (%) |
|---|---|---|---|
| CNC | 2394 ± 215 | 14.27 | 0.8 |
| CNC + 10% wt. IL | 1461 ± 74 | 14.31 | 0.7 |
| CNC + 25% wt. IL | 589 ± 290 | 4.65 | 1.0 |
| HPC | 919 ± 71 | 12.38 | 5.6 |
| MC | 1284 ± 183 | 15.26 | 2.8 |
| NaCMC | 1889 ± 323 | 21.27 | 4.3 |
| HPC + 40% wt. IL | 249 ± 45 | 2.52 | 14.1 |
| MC + 40% wt. IL | 570 ± 98 | 6.59 | 16.3 |
| NaCMC + 40% wt. IL | 208 ± 28 | 5.07 | 15.6 |
| Sample | Electrical Conductivity (S·m−1) | ||
|---|---|---|---|
| (i) | (ii) | (iii) | |
| CNC | 1.02 × 10−7 ± 0.0 | 3.82 × 10−9 ± 0.0 | 1.40 × 10−7 ± 0.0 |
| HPC | - | 2.42 × 10−7 ± 0.0 | - |
| MC | - | 2.22 × 10−8 ± 0.0 | - |
| NaCMC | 6.52 × 10−4 ± 0.0 | 2.17 × 10−5 ± 3.82 × 10−6 | 4.34 × 10−4 ± 3.87 × 10−5 |
| CNC + 10% wt. IL | 9.61 × 10−4 ± 1.40 × 10−4 | 4.39 × 10−5 ± 3.87 × 10−6 | 1.32 × 10−3 ± 6.53 × 10−5 |
| CNC + 25% wt. IL | 5.50 × 10−3 ± 1.18 × 10−4 | 1.29 × 10−4 ± 1.41 × 10−5 | 6.09 × 10−3 ± 1.22 × 10−4 |
| CNC + 40% wt. IL | 8.62 × 10−3 ± 3.45 × 10−4 | 1.21 × 10−4 ± 2.44 × 10−5 | 6.43 × 10−3 ± 3.53 × 10−4 |
| HPC + 40% wt. IL | - | 2.79 × 10−7 ± 3.99 × 10−8 | - |
| MC + 40% wt. IL | - | 5.75 × 10−8 ± 1.02 × 10−8 | - |
| NaCMC + 40% wt. IL | 1.25 × 10−3 ± 1.18 × 10−4 | 4.82 × 10−5 ± 0.0 | 2.37 × 10−3 ± 3.15 × 10−4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, D.M.; Lizundia, E.; Meira, R.M.; Rincón-Iglesias, M.; Lanceros-Méndez, S. Cellulose Nanocrystal and Water-Soluble Cellulose Derivative Based Electromechanical Bending Actuators. Materials 2020, 13, 2294. https://doi.org/10.3390/ma13102294
Correia DM, Lizundia E, Meira RM, Rincón-Iglesias M, Lanceros-Méndez S. Cellulose Nanocrystal and Water-Soluble Cellulose Derivative Based Electromechanical Bending Actuators. Materials. 2020; 13(10):2294. https://doi.org/10.3390/ma13102294
Chicago/Turabian StyleCorreia, Daniela M., Erlantz Lizundia, Rafaela M. Meira, Mikel Rincón-Iglesias, and Senentxu Lanceros-Méndez. 2020. "Cellulose Nanocrystal and Water-Soluble Cellulose Derivative Based Electromechanical Bending Actuators" Materials 13, no. 10: 2294. https://doi.org/10.3390/ma13102294