Interdisciplinary Methods for Zoonotic Tissue Acellularization for Natural Heart Valve Substitute of Biomimetic Materials
Abstract
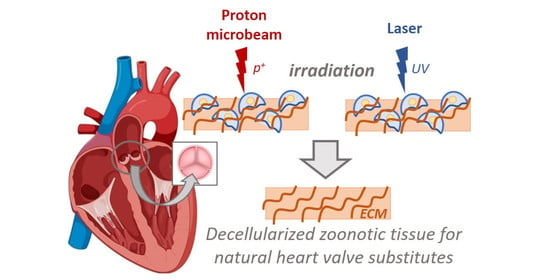
:1. Introduction
2. Materials and Methods
2.1. Tissue
2.2. Decellular Matrix Preparation
2.2.1. Fe Model of Decellularization Process
2.2.2. Decellularization Using Chemical Methods
2.2.3. Laser Method
Direct Laser Interference Lithography Experiments
Gaussian Beam Application
Top-Hat Beam
2.2.4. Proton Irradiation
2.3. Histology and Immunestaining
2.4. Principle of Analysis According to an Observer-Blind Mode
- assessment of optical densities for all techniques that were used to remove cells from tissue;
- percentage of image area not occupied by cells;
- number of cell nuclei per image;
- percentage of imaging area occupied by cell nuclei;
- average size of cell nuclei;
- circularity of cell nuclei.
3. Results
3.1. FE Modelling of Decellularization Process
3.2. Tissue Defect Analysis
3.3. Microscopic Analysis after Proton Irradiation
3.3.1. Laser Influence
3.3.2. Proton Radiation
3.3.3. Histology
Chemical Method
Laser
Protons
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herring, N.; Paterson, D.J. Levick’s Introduction to Cardiovascular Physiology, 6th ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Miller, G.W. King of Hearts; Crown Publishing Group: New York, NY, USA, 2010. [Google Scholar]
- Iaizzo, P.A. Handbook of Cardiac Anatomy, Physiology, and Devices; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, 72–227. [Google Scholar]
- Huang, G.; Rahimtoola, S.H. Prosthetic Heart Valve. Circulation 2011, 123, 2602–2605. [Google Scholar] [CrossRef] [PubMed]
- Lawley, C.M.; Lain, S.J.; Algert, C.S.; Ford, J.B.; Figtree, G.A.; Roberts, C.L. Prosthetic heart valves in pregnancy, outcomes for women and their babies: A systematic review and meta-analysis. BJOG 2015, 122, 1446–1455. [Google Scholar] [CrossRef] [Green Version]
- Toole, J.M.; Stroud, M.R.; Kratz, J.M.; Crumbley, A.J., 3rd; Bradley, S.M.; Crawford, A.F., Jr.; Ikonomidis, J.S. Twenty-five year experience with the St. Jude medical mechanical valve prosthesis. Ann. Thorac. Surg. 2010, 89, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Tesar, P.J.; Pearse, B.; Jalali, H.; Sparks, L.; Fraser, J.F.; Pohlner, P.G. Long-term clinical outcomes after aortic valve replacement using cryopreserved aortic allograft. J. Thorac. Cardiovasc. Surg. 2014, 148, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Emmert, M.Y.; Weber, B.; Behr, L.; Sammut, S.; Frauenfelder, T.; Wolint, P.; Scherman, J.; Bettex, D.; Grünenfelder, J.; Falk, V.; et al. Transcatheter aortic valve implantation using anatomically oriented, marrow stromal cell-based, stented, tissue-engineered heart valves: Technical considerations and implications for translational cell-based heart valve concepts. Eur. J. Cardiothorac. Surg. 2014, 45, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Gastélum, G.R.; Aguilar-Medina, E.M.; Soto-Sainz, E. Antimicrobial Properties of Extracellular Matrix Scaffolds for Tissue Engineering. Biomed. Res. Int. 2019, 2019, 9641456. [Google Scholar] [CrossRef]
- Sicari, B.M.; Londono, R.L.; Badylak, S.F. Extracellular Matrix as a Bioscaffold for Tissue Engineering. In Tissue Engineering, 2nd ed.; Van Blitterswijk, C.A., De Boer, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 149–176. [Google Scholar]
- Cramer, M.; Chang, J.; Li, H.; Serrero, A.; El-Kurdi, M.; Cox, M.; Schoen, F.J.; Badylak, S.F. Tissue response, macrophage phenotype, and intrinsic calcification induced by cardiovascular biomaterials: Can clinical regenerative potential be predicted in a rat subcutaneous implant model? J. Biomed. Mater. Res. A 2022, 110, 245–256. [Google Scholar] [CrossRef]
- Costa, A.; Naranjo, J.D.; Londono, R.; Badylak, S.F. Biologic Scaffolds. Cold Spring Harb. Perspect. Med. 2017, 7, a025676. [Google Scholar] [CrossRef] [Green Version]
- Huleihel, L.; Dziki, J.L.; Bartolacci, J.G.; Rausch, T.; Scarritt, M.E.; Cramer, M.C.; Vorobyov, T.; LoPresti, S.T.; Swineheart, I.T.; White, L.; et al. Macrophage phenotype in response to ECM bioscaffolds. Semin. Immunol. 2017, 29, 2–13. [Google Scholar] [CrossRef]
- Urbanski, W.; Marycz, K.; Krzak, J.; Pezowicz, C.; Dragan, S.F. Cytokine induction of sol-gel-derived TiO2 and SiO2 coatings on metallic substrates after implantation to rat femur. Int. J. Nanomed. 2017, 12, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Vitanova, K.; Wirth, F.; Boehm, J.; Burri, M.; Lange, R.; Krane, M. Surgical Aortic Valve Replacement-Age-Dependent Choice of Prosthesis Type. J. Clin. Med. 2021, 10, 5554. [Google Scholar] [CrossRef] [PubMed]
- VanderLaan, P.A.; Padera, R.F.; Schoen, F.J. Practical Approach to the Evaluation of Prosthetic Mechanical and Tissue Replacement Heart Valves. Surg. Pathol. Clin. 2012, 5, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.H.S.; Wender, O.C.B.; de Almeida, A.S.; Soares, L.E.; Picon, P.D. Comparison of clinical outcomes in patients undergoing mitral valve replacement with mechanical or biological substitutes: A 20 years cohort. BMC Cardiovasc. Disord. 2014, 14, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajab, T.K.; Ali, J.M.; Hernández-Sánchez, J.; Mackie, J.; Grimaudo, V.; Sinichino, S.; Mills, C.; Rana, B.; Dunning, J.; Abu-Omar, Y. Mid-term follow-up after aortic valve replacement with the Carpentier Edwards Magna Ease prosthesis. J. Cardiothorac. Surg. 2020, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, L.; Jacques, N.; Hego, A.; Nchimi, A.; Lancellotti, P.; Oury, C. Prosthetic Aortic Valves: Challenges and Solutions. Front. Cardiovasc. Med. 2018, 5, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadori, M.; Cozzi, E. The immunological barriers to xenotransplantation. Tissue Antigens 2015, 86, 239–253. [Google Scholar] [CrossRef]
- Cooper, K.C.; Byrne, G. Clinical Xenotransplantation; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Galili, U. Anti-Gal: An abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology 2013, 140, 1–11. [Google Scholar] [CrossRef]
- Tianyu, L.; Bochao, Y.; Ruolin, W.; Chuan, Q. Xenotransplantation: Current Status in Preclinical Research. Front. Immunol. 2020, 10, 3060. [Google Scholar]
- Tam, H.; Zhang, W.; Infante, D.; Parchment, N.; Sacks, M.; Vyavahare, N. Fixation of Bovine Pericardium-Based Tissue Biomaterial with Irreversible Chemistry Improves Biochemical and Biomechanical Properties. J. Cardiovasc. Transl. Res. 2017, 10, 194–205. [Google Scholar] [CrossRef]
- Kostyunin, A.E.; Yuzhalin, A.E.; Rezvova, M.A.; Ovcharenko, E.; Glushkova, T.V.; Kutikhin, A.G. Degeneration of Bioprosthetic Heart Valves: Update 2020. JAHA 2020, 9, e018506. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Goergen, C.J.; Schoen, F.J.; Aikawa, M.; Zilla, P.; Aikawa, E.; Gaudette, G.R. After 50 Years of Heart Transplants: What Does the Next 50 Years Hold for Cardiovascular Medicine? A Perspective from the International Society for Applied Cardiovascular Biology. Front. Cardiovasc. Med. 2019, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Wilczek, P. Heart valve bioprothesis; effect of different acellularizations methods on the biomechanical and morphological properties of porcine aortic and pulmonary valve. Bull. Pol. Acad. Sci. 2010, 58, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef]
- Nagata, S.; Hanayama, R.; Kawane, K. Autoimmunity and the clearance of dead cells. Cell 2010, 140, 619–630. [Google Scholar] [CrossRef] [Green Version]
- Devi, K.S.; Anandasabapathy, N. The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin. Immunopathol. 2017, 39, 137–152. [Google Scholar] [CrossRef]
- Niu, Y.; Stadler, F.J.; Yang, X.; Deng, F.; Liu, G.; Xia, H. HA-coated collagen nanofibers for urethral regeneration via in situ polarization of M2 macrophages. J. Nanobiotechnol. 2021, 19, 283. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Mathapati, S.; Bishi, D.K.; Venugopal, J.R.; Cherian, K.M.; Guhathakurta, S.; Ramakrishna, S.; Verma, R.S. Nanofibers coated on acellular tissue-engineered bovine pericardium supports differentiation of mesenchymal stem cells into endothelial cells for tissue engineering. Nanomedicine 2014, 9, 623–634. [Google Scholar] [CrossRef]
- Cui, H.; Chai, Y.; Yu, Y. Progress in developing decellularized bioscaffolds for enhancing skin construction. J. Biomed. Mater. Res. Part A 2019, 107, 1849–1859. [Google Scholar] [CrossRef]
- Reing, J.; Brown, B.N.; Daly, K.A.; Freund, J.M.; Gilbert, T.; Hsiong, S.X.; Huber, A.; Kullas, K.E.; Tottey, S.; Wolf, M.T.; et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 2010, 31, 8626–8633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elder, B.D.; Kim, D.H.; Athanasiou, K.A. Developing an articular cartilage decellularization process toward facet joint cartilage replacement. Neurosurgery 2010, 66, 722–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Wu, X.; Pang, K.; Yang, Y. Histological evaluation and biomechanical characterisation of an acellular porcine cornea scaffold. Br. J. Ophthalmol. 2010, 95, 410–414. [Google Scholar] [CrossRef]
- Trindade, D.; Cordeiro, R.; José, H.C.; Ângelo, D.F.; Alves, N.; Moura, C. Biological Treatments for Temporomandibular Joint Disc Disorders: Strategies in Tissue Engineering. Biomolecules 2021, 11, 933. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, Y.; Zhou, L.; Sun, Z.; Zheng, J.; Chen, Y.; Dai, Y. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng C 2010, 16, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Clippinger, B.; Conrad, C.; Schuetz, C.; Pomerantseva, I.; Ikonomou, L. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 2010, 16, 927–933. [Google Scholar] [CrossRef]
- Funamoto, S.; Nam, K.; Kimura, T.; Murakoshi, A.; Hashimoto, Y.; Niwaya, K.; Kitamura, S.; Fujisato, T.; Kishida, A. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials 2010, 31, 3590–3595. [Google Scholar] [CrossRef]
- Deeken, C.R.; White, A.K.; Bachman, S.L.; Ramshaw, B.J.; Cleveland, D.S.; Loy, T.S.; Grant, S.A. Method of preparing a decellularized porcine tendon using tributyl phosphate. J. Biomed. Mater. Res. B 2010, 96, 199–206. [Google Scholar] [CrossRef]
- Walkden, A. Amniotic Membrane Transplantation in Ophthalmology: An Updated Perspective. Clin. Ophthalmol. 2020, 22, 2057–2072. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, S.; Ding, J.; Xu, J.; Shi, J.; Dong, N. Tissue engineering of heart valves: PEGylation of decellularized porcine aortic valve as a scaffold for in vitro recellularization. BioMed. Eng. OnLine 2013, 12, 87. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Fritze, O.; Schleicher, M.; Wendel, H.P.; Schenke-Layland, K.; Harasztosi, C. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials 2010, 31, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Ackbar, R.; Ainoedhofer, H.; Gugatschka, M.; Saxena, A.K. Decellularized ovine esophageal mucosa for esophageal tissue engineering. Technol. Health Care 2012, 20, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Isidan, A.; Liu, S.; Li, P.; Lashmet, M.; Smith, L.J.; Hara, H.; Cooper, D.K.C.; Ekser, B. Decellularization methods for developing porcine corneal xenografts and future perspectives. Xenotransplantation 2019, 26, e12564. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Funamoto, S.; Sasaki, S.; Honda, T.; Hattori, S.; Nam, K. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials 2010, 31, 3941–3948. [Google Scholar] [CrossRef]
- Song, L.; Murphy, S.V.; Yang, B.; Xu, Y.; Zhang, Y.; Atala, A. Bladder acellular matrix and its application in bladder augmentation. Tissue Eng. Part B Rev. 2014, 20, 163–172. [Google Scholar] [CrossRef]
- Rabbani, M.; Zakian, N.; Aimoradi, N. Contribution of physical methods in decellularization of animal tissues. J. Med. Signals Sens. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Scarritt, M.E.; Pashos, N.C.; Bunnell, B.A. A review of cellularization strategies for tissue engineering of whole organs. Front. Bioeng. Biotechnol. 2015, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Kopernik, M.; Major, R.; Lis, G.; Wilczek, P.; Lis, M.; Imbir, G.; Chrouda, A.; Mzyk, A.; Ostrowski, R.; Sanak, M. The interaction of laser radiation with tissue in the aspect of generating the process of decellularization in the preparation of animal origin autologous tissue. Acta Bioeng. Biomech. 2020, 22, 67–77. [Google Scholar] [CrossRef]
- Kopernik, M.; Milenin, A.; Major, R.; Lackner, J.M. Identification of material model of TiN using numerical simulation of nanoindentation test. Mater. Sci. Technol. 2011, 27, 604–616. [Google Scholar]
- Hamdan, A.; Guetta, V.; Konen, E.; Goitein, O.; Segev, A.; Raanani, E.; Spiegelstein, D.; Hay, I.; Di Segni, E.; Eldar, M.; et al. Deformation Dynamics and Mechanical Properties of the Aortic Annulus by 4-Dimensional Computed Tomography: Insights in to the Functional Anatomy of the Aortic Valve Complex and Implications for Transcatheter Aortic Valve Therapy. J. Am. Coll. Cardiol. 2012, 59, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Czerner, M.; Sanchez-Fellay, L.; Suarez, M.P.; Frontini, P.M.; Fasce, L.A. Determination of Elastic Modulus of Gelatin Gels by Indentation Experiments. Procedia Mater. Sci. 2015, 8, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Korossis, S. Structure-Function Relationship of Heart Valves in Health and Disease. Structural Insufficiency Anomalies in Cardiac Valves; IntechOpen: London, UK, 2018. [Google Scholar]
- Chen, J.H.; Simmons, C.A.; Towler, D.A. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: Critical roles for matricellular, matricrine, and matrix mechanics cues. Circ. Res. 2011, 108, 1510–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazir, R.; Bruyneel, A.; Carr, C.; Czernuszka, J. Mechanical and Degradation Properties of Hybrid Scaffolds for Tissue Engineered Heart Valve (TEHV). J. Funct. Biomater. 2021, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Marczak, J.; Rycyk, A.; Sarzyński, A.; Strzelec, M.; Czyż, K. An Nd:YAG dual-channel laser system with Q—Modulation for direct interference lithography. Photon-Lett. Pol. 2014, 6, 44–46. [Google Scholar]
- Wilczek, P.; Major, R.; Ostrowski, R.; Strzelec, M.; Rycyk, A. A Method of Decellularization of Collagen Tissues, Especially Heart Valve Tissues. PL Patent No. P.426790, 27 August 2018. The Patent Office of the Republic of Poland: Warsaw, Poland, 2018. Available online: https://ewyszukiwarka.pue.uprp.gov.pl/search/pwp-details/P.426790 (accessed on 10 March 2022).
- Durante, M.; Orecchia, R.; Loeffler, J.S. Charged-particle therapy in cancer: Clinical uses and future perspectives. Nat. Rev. Clin. Oncol. 2017, 14, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Lebed, S.; Stachura, Z.; Cholewa, M.; Legge, G.J.F.; Lekki, J.; Maranda, S.; Potempa, A.; Sarnecki, C.; Szklarz, Z.; Styczen, J.; et al. The new Cracow scanning nuclear microprobe. Nucl. Instrum. Methods Phys. Res. Sect. B 2001, 181, 95–98. [Google Scholar] [CrossRef]
- König, K.; Schenke-Layland, K.; Riemann, I.; Stock, U.A. Multiphoton autofluorescence imaging of intratissue elastic fibers. Biomaterials 2005, 26, 495–500. [Google Scholar] [CrossRef]
- Baugh, L.M.; Liu, Z.; Quinn, K.P.; Osseiran, S.; Evans, C.L.; Huggins, G.S.; Hinds, P.W.; Black, L.D., 3rd; Georgakoudi, I. Non-destructive two-photon excited fluorescence imaging identifies early nodules in calcific aortic-valve disease. Nat. Biomed. Eng. 2017, 11, 914–924. [Google Scholar] [CrossRef] [Green Version]
- Litvinova, K.; Chernysheva, M.; Stegemann, B.; Leyva, F. Autofluorescence guided welding of heart tissue by laser pulse bursts at 1550 nm. Biomed. Opt. Express 2020, 11, 6271–6280. [Google Scholar] [CrossRef]
- Gayen, T.K.; Katz, A.; Savage, H.E.; McCormick, S.A.; Al-Rubaiee, M.; Budansky, Y.; Lee, J.; Alfano, R.R. Aorta and skin tissues welded by near-infrared Cr4+: YAG laser. J. Clin. Laser Med. Surg. 2003, 21, 259–269. [Google Scholar] [CrossRef]
- Koch, R.G.; Tsamis, A.; D’Amore, A.; Wagner, W.R.; Watkins, S.C.; Gleason, T.G.; Vorp, D.A. A custom image-based analysis tool for quantifying elastin and collagen micro-architecture in the wall of the human aorta from multi-photon microscopy. J. Biomech. 2014, 47, 935–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, L.A.; Chiu, L.L.; Feric, N.; Fu, L.; Radisic, M. Biomaterials in myocardial tissue engineering. J. Tissue Eng. Regen. Med. 2016, 10, 11–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, F.; Bronk, L.; Titt, U.; Lin, S.H.; Mirkovic, D.; Kerr, M.D.; Zhu, X.R.; Dinh, J.; Sobieski, M.; Stephan, C.; et al. Spatial mapping of the biologic effectiveness of scanned particle beams: Towards biologically optimized particle therapy. Sci. Rep. 2015, 5, 9850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, V.; Wilson, M.W. DNA Damage and Associated DNA Repair Defects in Disease and Premature Aging. Am. J. Hum. Genet. 2019, 105, 237–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, T.E.; Canella, L.; Kudejova, P.; Wagner, F.M.; Röhrmoser, A.; Schmid, E. The effectiveness of the high-LET radiations from the boron neutron capture [10B(n,α) 7Li] reaction determined for induction of chromosome aberrations and apoptosis in lymphocytes of human blood samples. Radiat. Environ. Biophys. 2015, 54, 91–102. [Google Scholar] [CrossRef]
- Steen, W.M.; Mazumder, J. Laser Material Processing, 4th ed.; Springer: London, UK, 2010. [Google Scholar]
- Bäuerle, D. Laser Processing and Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Vogel, A.; Venugopalan, V. Pulsed Laser Ablation of Soft Biological Tissues. In Optical-Thermal Response of Laser-Irradiated Tissue, 2nd ed.; Welch, A.J., van Gemert, M.J.C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 551–615. [Google Scholar]
- Syazwani, N.; Shafiq, M.; Ushida, T.; Azhim, A. Simulation and experimental measurement of acoustic intensity on sonication parameters and decellularization using sonication treatment. J. Signal Process. 2015, 19, 179–182. [Google Scholar] [CrossRef] [Green Version]















| Tissue | Young’s Modulus (MPa) | Hyperelastic Properties | |
|---|---|---|---|
| Fibrosa | 1.841 | Ogden model, N = 1 | |
| μ1 = 0.008 | α1 = 25 | ||
| Spongiosa | 0.809 | Ogden model, N = 3 | |
| μ1 = −0.326 | α1 = 14.675 | ||
| μ2 = 0.298 | α2 = 14.796 | ||
| μ3 = 0.043 | α3 = 11.981 | ||
| Ventricularis | 1.622 | Ogden model, N = 3 | |
| μ1 = −18.610 | α1 = 18.231 | ||
| μ2 = 10.025 | α2 = 18.778 | ||
| μ3 = 8.606 | α3 = 17.532 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Major, R.; Kopernik, M.; Ostrowski, R.; Wilczek, P.; Bartkowiak, A.; Szawiraacz, K.; Lis, G.; Lekki, J.; Gawlikowski, M.; Major, Ł. Interdisciplinary Methods for Zoonotic Tissue Acellularization for Natural Heart Valve Substitute of Biomimetic Materials. Materials 2022, 15, 2594. https://doi.org/10.3390/ma15072594
Major R, Kopernik M, Ostrowski R, Wilczek P, Bartkowiak A, Szawiraacz K, Lis G, Lekki J, Gawlikowski M, Major Ł. Interdisciplinary Methods for Zoonotic Tissue Acellularization for Natural Heart Valve Substitute of Biomimetic Materials. Materials. 2022; 15(7):2594. https://doi.org/10.3390/ma15072594
Chicago/Turabian StyleMajor, Roman, Magdalena Kopernik, Roman Ostrowski, Piotr Wilczek, Amanda Bartkowiak, Karolina Szawiraacz, Grzegorz Lis, Janusz Lekki, Maciej Gawlikowski, and Łukasz Major. 2022. "Interdisciplinary Methods for Zoonotic Tissue Acellularization for Natural Heart Valve Substitute of Biomimetic Materials" Materials 15, no. 7: 2594. https://doi.org/10.3390/ma15072594