Multi-Parametric Exploration of a Selection of Piezoceramic Materials for Bone Graft Substitute Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Piezoceramic Materials
- BaTiO3 (BT)—reagents: BaCO3 and TiO2;
- Zr-doped (2 mol%) BT (Zr:BT)—reagents: BaCO3, TiO2 and ZrO2;
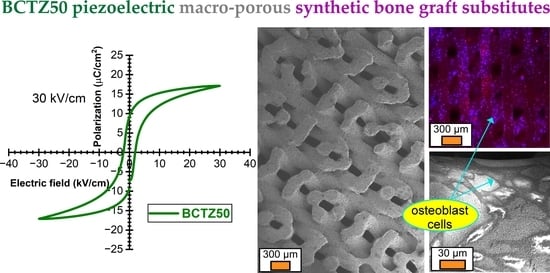
- (Ba0.85Ca0.15)(Ti0.9Zr0.1)O3 solid solution (BCTZ50)—reagents: BaCO3, CaCO3, TiO2 and ZrO2;
- KNbO3 (KNO)—reagents: K2CO3 and Nb2O5;
- LiNbO3 (LNO)—reagents: Li2CO3 and Nb2O5;
- LiTaO3 (LTO)—reagents: Li2CO3 and Ta2O5.
2.2. Fabrication of Piezoceramic Macro-Porous Scaffolds by Robocasting
2.3. Structural, Morphological, Electrical, and Mechanical Characterization
2.4. In Vitro Biological Assays
2.4.1. Sintered Disks
2.4.2. Macro-Porous Scaffolds
2.4.3. Statistical Significance Analysis
3. Results and Discussion
3.1. Multi-Parametric Analysis of the Sintered Disks
3.1.1. Structural, Density and Morphological Evaluation
3.1.2. Dielectric and Piezoelectric Properties
3.1.3. In Vitro (pH and Cell Compatibility) Response
3.2. Translation to Real Biomedical Applications—Pilot Studies of BCTZ Macro-Porous Bone Scaffolds
3.2.1. Morphological Analyses
3.2.2. Compressive Strength Performance
3.2.3. Cytocompatibility Assessments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, R.; Bishop, T.; Valerio, I.L.; Fisher, J.P.; Dean, D. The potential impact of bone tissue engineering in the clinic. Regen. Med. 2016, 11, 571–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, G.; Liang, H.; Gao, C.; Peng, S.; Shen, L.; Shuai, C. Additive manufacturing of bone scaffolds. Int. J. Bioprinting 2018, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Life Expencancy and Healthy Life Expectancy. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy (accessed on 28 November 2022).
- Ebrahimi, M.; Botelho, M.G.; Dorozhkin, S.V. Biphasic calcium phosphates bioceramics (HA/TCP): Concept, physicochemical properties and the impact of standardization of study protocols in biomaterials research. Mater. Sci. Eng. C 2017, 71, 1293–1312. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.; Becerro, A.; Pardal-Peláez, B.; Quispe-López, N.; Blanco, J.-F.; Gómez-Polo, C. Main 3D manufacturing techniques for customized bone substitutes. A systematic review. Materials 2021, 14, 2524. [Google Scholar] [CrossRef] [PubMed]
- Pedrero, S.G.; Llamas-Sillero, P.; Serrano-López, J. A multidisciplinary journey towards bone tissue engineering. Materials 2021, 14, 4896. [Google Scholar] [CrossRef]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. 3D-printed hydroxyapatite and tricalcium phosphates-based scaffolds for alveolar bone regeneration in animal models: A scoping review. Materials 2022, 15, 2621. [Google Scholar] [CrossRef]
- Zaszczyńska, A.; Moczulska-Heljak, M.; Gradys, A.; Sajkiewicz, P. Advances in 3D printing for tissue engineering. Materials 2021, 14, 3149. [Google Scholar] [CrossRef]
- Hannink, G.; Arts, J.J.C. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef]
- Grand View Research. Bone Grafts and Substitutes Market Size, Share & Trends Analysis. Available online: https://www.grandviewresearch.com/industry-analysis/bone-grafts-substitutes-market (accessed on 28 November 2022).
- Precedence Research. Bone Grafts and Substitutes Market. Available online: https://www.precedenceresearch.com/bone-grafts-and-substitutes-market (accessed on 28 November 2022).
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Amini, Z.; Lari, R. A systematic review of decellularized allograft and xenograft-derived scaffolds in bone tissue regeneration. Tissue Cell 2021, 69, 101494. [Google Scholar] [CrossRef]
- Bracey, D.N.; Cignetti, N.E.; Jinnah, A.H.; Stone, A.V.; Gyr, B.M.; Whitlock, P.W.; Scott, A.T. Bone xenotransplantation: A review of the history, orthopedic clinical literature, and a single-center case series. Xenotransplantation 2020, 27, 12600. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone grafts and substitutes in dentistry: A review of current trends and developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef]
- Gritsch, L.; Maqbool, M.; Mouriño, V.; Ciraldo, F.E.; Cresswell, M.; Jackson, P.R.; Lovell, C.; Boccaccini, A.R. Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: Copper and strontium. J. Mater. Chem. B 2019, 7, 6109–6124. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Hamdi, M.; Basirun, W.J. Bioglass® 45S5-based composites for bone tissue engineering and functional applications. J. Biomed. Mater. Res. Part A 2017, 105, 3197–3223. [Google Scholar] [CrossRef]
- Lu, J.; Yu, H.; Chen, C. Biological properties of calcium phosphate biomaterials for bone repair: A review. RSC Adv. 2018, 8, 2015–2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besleaga, C.; Nan, B.; Popa, A.-C.; Balescu, L.M.; Nedelcu, L.; Neto, A.S.; Pasuk, I.; Leonat, L.; Popescu-Pelin, G.; Ferreira, J.M.F.; et al. Sr and Mg doped bi-phasic calcium phosphate macroporous bone graft substitutes fabricated by robocasting: A structural and cytocompatibility assessment. J. Funct. Biomater. 2022, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Mirzaali, M.J.; Schwiedrzik, J.J.; Thaiwichai, S.; Best, J.P.; Michler, J.; Zysset, P.K.; Wolfram, U. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone 2016, 93, 196–211. [Google Scholar] [CrossRef]
- Li, J.; Bao, Q.; Chen, S.; Liu, H.; Feng, J.; Qin, H.; Li, A.; Liu, D.; Shen, Y.; Zhao, Y.; et al. Different bone remodeling levels of trabecular and cortical bone in response to changes in Wnt/β-catenin signaling in mice. J. Orthop. Res. 2017, 35, 812–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, Y.; Takao, H.; Tani, T.; Nonoyama, T.; Takatori, K.; Homma, T.; Nagaya, T.; Nakamura, M. Lead-free piezoceramics. Nature 2004, 432, 84–87. [Google Scholar] [CrossRef]
- Ioachim, A.; Alexandru, H.V.; Berbecaru, C.; Antohe, S.; Stanculescu, F.; Banciu, M.G.; Toacsen, M.I.; Nedelcu, L.; Ghetu, D.; Dutu, A.; et al. Dopant influence on BST ferroelectric solid solutions family. Mater. Sci. Eng. C 2006, 26, 1156–1161. [Google Scholar] [CrossRef]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm. Regen. 2018, 38, 2. [Google Scholar] [CrossRef] [Green Version]
- Jarkov, V.; Allan, S.J.; Bowen, C.; Khanbareh, H. Piezoelectric materials and systems for tissue engineering and implantable energy harvesting devices for biomedical applications. Int. Mater. Rev. 2022, 67, 683–733. [Google Scholar] [CrossRef]
- Fukada, E.; Yasuda, I. On the piezoelectric effect of bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Ulstrup, A.K. Biomechanical concepts of fracture healing in weight-bearing long bones. Acta Orthop. Belg. 2008, 74, 291–302. [Google Scholar]
- Uto, Y.; Kuroshima, S.; Nakano, T.; Ishimoto, T.; Inaba, N.; Uchida, Y.; Sawase, T. Effects of mechanical repetitive load on bone quality around implants in rat maxillae. PLoS ONE 2017, 12, e0189893. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Ning, C.; Zhang, Y.; Tan, G.; Lin, Z.; Liu, S.; Wang, X.; Yang, H.; Li, K.; Yi, X.; et al. Bone-inspired spatially specific piezoelectricity induces bone regeneration. Theranostics 2017, 7, 3387–3397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilarinho, P.M.; Barroca, N.; Zlotnik, S.; Félix, P.; Fernandes, M.H. Are lithium niobate (LiNbO3) and lithium tantalate (LiTaO3) ferroelectrics bioactive? Mater. Sci. Eng. C 2014, 39, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Carville, N.C.; Collins, L.; Manzo, M.; Gallo, K.; Lukasz, B.I.; McKayed, K.K.; Simpson, J.C.; Rodriguez, B.J. Biocompatibility of ferroelectric lithium niobate and the influence of polarization charge on osteoblast proliferation and function. J. Biomed. Mater. Res. Part A 2015, 103, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Danti, S.; D’Alessandro, D.; Moscato, S.; Petrini, M.; Menciassi, A. Barium titanate nanoparticles: Highly cytocompatible dispersions in glycol-chitosan and doxorubicin complexes for cancer therapy. Nanoscale Res. Lett. 2010, 5, 1093–1101. [Google Scholar] [CrossRef] [Green Version]
- Candito, M.; Simoni, E.; Gentilin, E.; Martini, A.; Marioni, G.; Danti, S.; Astolfi, L. Neuron compatibility and antioxidant activity of barium titanate and lithium niobate nanoparticles. Int. J. Mol. Sci. 2022, 23, 1761. [Google Scholar] [CrossRef]
- Saranya, K.; Thirupathi Kumara Raja, S.; Subhasree, R.S.; Gnanamani, A.; Das, S.K.; Rajendran, N. Fabrication of nanoporous sodium niobate coating on 316L SS for orthopaedics. Ceram. Int. 2017, 43, 11569–11579. [Google Scholar] [CrossRef]
- Yu, S.W.; Kuo, S.T.; Tuan, W.H.; Tsai, Y.Y.; Wang, S.F. Cytotoxicity and degradation behavior of potassium sodium niobate piezoelectric ceramics. Ceram. Int. 2012, 38, 2845–2850. [Google Scholar] [CrossRef]
- Mancuso, E.; Shah, L.; Jindal, S.; Serenelli, C.; Tsikriteas, Z.M.; Khanbareh, H.; Tirella, A. Additively manufactured BaTiO3 composite scaffolds: A novel strategy for load bearing bone tissue engineering applications. Mater. Sci. Eng. C 2021, 126, 112192. [Google Scholar] [CrossRef]
- Shimada, S.; Kodaira, K.; Matsushita, T. Sintering LiTaO3 and KTaO3 with the aid of manganese oxide. J. Mater. Sci. 1984, 19, 1385–1390. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Popa, A.C.; Marques, V.M.F.; Stan, G.E.; Husanu, M.A.; Galca, A.C.; Ghica, C.; Tulyaganov, D.U.; Lemos, A.F.; Ferreira, J.M.F. Nanomechanical characterization of bioglass films synthesized by magnetron sputtering. Thin Solid Film. 2014, 553, 166–172. [Google Scholar] [CrossRef]
- Popa, A.C.; Stan, G.E.; Besleaga, C.; Ion, L.; Maraloiu, V.A.; Tulyaganov, D.U.; Ferreira, J.M.F. Submicrometer hollow bioglass cones deposited by radio frequency magnetron sputtering: Formation mechanism, properties, and prospective biomedical applications. ACS Appl. Mater. Interfaces 2016, 8, 4357–4367. [Google Scholar] [CrossRef]
- Besleaga, C.; Dumitru, V.; Trinca, L.M.; Popa, A.C.; Negrila, C.C.; Kołodziejczyk, Ł.; Luculescu, C.R.; Ionescu, G.C.; Ripeanu, R.G.; Vladescu, A.; et al. Mechanical, corrosion and biological properties of room-temperature sputtered aluminum nitride films with dissimilar nanostructure. Nanomaterials 2017, 7, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stan, G.E.; Tite, T.; Popa, A.-C.; Chirica, I.M.; Negrila, C.C.; Besleaga, C.; Zgura, I.; Sergentu, A.C.; Popescu-Pelin, G.; Cristea, D.; et al. The beneficial mechanical and biological outcomes of thin copper-gallium doped silica-rich bio-active glass implant-type coatings. Coatings 2020, 10, 1119. [Google Scholar] [CrossRef]
- Liu, W.; Ren, X. Large piezoelectric effect in Pb-free ceramics. Phys. Rev. Lett. 2009, 103, 257602. [Google Scholar] [CrossRef] [Green Version]
- Keeble, D.S.; Benabdallah, F.; Thomas, P.A.; Maglione, M.; Kreisel, J. Revised structural phase diagram of (Ba0.7Ca0.3TiO3)-(BaZr0.2Ti0.8O3). Appl. Phys. Lett. 2013, 102, 92903. [Google Scholar] [CrossRef]
- Hao, J.; Bai, W.; Li, W.; Zhai, J. Correlation between the microstructure and electrical properties in high-performance (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 lead-free piezoelectric ceramics. J. Am. Ceram. Soc. 2012, 95, 1998–2006. [Google Scholar] [CrossRef]
- Cao, W.; Randall, C.A. Grain size and domain size relations in bulk ceramic ferroelectric materials. J. Phys. Chem. Solids 1996, 57, 1499–1505. [Google Scholar] [CrossRef]
- Arlt, G. The influence of microstructure on the properties of ferroelectric ceramics. Ferroelectrics 1990, 104, 217–227. [Google Scholar] [CrossRef]
- Yang, T.; Liu, Y.G.; Zhang, L.; Hu, M.L.; Yang, Q.; Huang, Z.H.; Fang, M.H. Powder synthesis and properties of LiTaO3 ceramics. Adv. Powder Technol. 2014, 25, 933–936. [Google Scholar] [CrossRef]
- Diaz-Moreno, C.A.; Ding, Y.; Portelles, J.; Heiras, J.; Macias, A.H.; Syeed, A.; Paez, A.; Li, C.; López, J.; Wicker, R. Optical properties of ferroelectric lanthanum lithium niobate. Ceram. Int. 2018, 44, 4727–4733. [Google Scholar] [CrossRef]
- Damjanovic, D.; Biancoli, A.; Batooli, L.; Vahabzadeh, A.; Trodahl, J. Elastic, dielectric, and piezoelectric anomalies and Raman spectroscopy of 0.5Ba(Ti0.8Zr0.2)O3-0.5(Ba0.7Ca0.3)TiO3. Appl. Phys. Lett. 2012, 100, 192907. [Google Scholar] [CrossRef] [Green Version]
- Trzepiecinski, T.; Gromada, M. Characterization of mechanical properties of barium titanate ceramics with different grain sizes. Mater. Sci. 2018, 36, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Coondoo, I.; Panwar, N.; Alikin, D.; Bdikin, I.; Islam, S.S.; Turygin, A.; Shur, V.Y.; Kholkin, A.L. A comparative study of structural and electrical properties in lead-free BCZT ceramics: Influence of the synthesis method. Acta Mater. 2018, 155, 331–342. [Google Scholar] [CrossRef]
- Tsui, T.Y.; Pharr, G.M.; Oliver, W.C.; Bhatia, C.S.; White, R.L.; Anders, S.; Anders, A.; Brown, I.G. Nanoindentation and nanoscratching of hard carbon coatings for magnetic disks. MRS Proc. 1995, 383, 447. [Google Scholar] [CrossRef] [Green Version]
- Tite, T.; Popa, A.C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic substitutions in hydroxyapatite: Current status of the derived biofunctional effects and their in vitro interrogation methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef] [Green Version]
- Toxicological Profile for Barium and Barium Compounds. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp24-c2.pdf (accessed on 28 November 2022).
- Bansod, Y.D.; Kebbach, M.; Kluess, D.; Bader, R.; van Rienen, U. Finite element analysis of bone remodelling with piezoelectric effects using an open-source framework. Biomech. Model. Mechanobiol. 2021, 20, 1147–1166. [Google Scholar] [CrossRef]
- Bansod, Y.D.; Kebbach, M.; Kluess, D.; Bader, R.; van Rienen, U. Computational analysis of bone remodeling in the proximal tibia under electrical stimulation considering the piezoelectric properties. Front. Bioeng. Biotechnol. 2021, 9, 705199. [Google Scholar] [CrossRef]
- Carter, A.; Popowski, K.; Cheng, K.; Greenbaum, A.; Ligler, F.S.; Moatti, A. Enhancement of bone regeneration through the converse piezoelectric effect. A novel approach for applying mechanical stimulation. Bioelectricity 2021, 3, 255–271. [Google Scholar] [CrossRef]
- Fernández, J.R.; García-Aznar, J.M.; Martínez, R. Piezoelectricity could predict sites of formation/resorption in bone remodelling and modelling. J. Theor. Biol. 2012, 292, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Schult, M.; Buckow, E.; Seitz, H. Experimental studies on 3D printing of barium titanate ceramics for medical applications. Curr. Dir. Biomed. Eng. 2016, 2, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Tariverdian, T.; Behnamghader, A.; Brouki Milan, P.; Barzegar-Bafrooei, H.; Mozafari, M. 3D-printed barium strontium titanate-based piezoelectric scaffolds for bone tissue engineering. Ceram. Int. 2019, 45, 14029–14038. [Google Scholar] [CrossRef]
- Nan, B.; Olhero, S.; Pinho, R.; Vilarinho, P.M.; Button, T.W.; Ferreira, J.M.F. Direct ink writing of macroporous lead-free piezoelectric Ba0.85Ca0.15Zr0.1Ti0.9O3. J. Am. Ceram. Soc. 2019, 102, 3191–3203. [Google Scholar] [CrossRef]
- Sugimoto, H.; Biggemann, J.; Fey, T.; Singh, P.; Khare, D.; Dubey, A.K.; Kakimoto, K. Lead-free piezoelectric (Ba,Ca)(Ti,Zr)O3 scaffolds for enhanced antibacterial property. Mater. Lett. 2021, 297, 129969. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Li, B. Direct ink writing of three-dimensional (K, Na)NbO3-based piezoelectric ceramics. Materials 2015, 8, 1729–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Fu, J.; Xu, Y.; Zuo, R. Field-insensitive giant dynamic piezoelectric response and its structural origin in (Ba,Ca)(Ti,Zr)O3 tetragonal-orthorhombic phase-boundary ceramics. J. Eur. Ceram. Soc. 2021, 41, 6441–6448. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Shi, W.; Wang, Q.; jiang, G.; Yang, B.; Cao, W.; Tan, J. Ultrahigh strain in textured BCZT-based lead-free ceramics with CuO sintering agent. J. Mater. Sci. Technol. 2022, 117, 207–214. [Google Scholar] [CrossRef]
- Hayati, R.; Bahrevar, M.A.; Ganjkhanlou, Y.; Rojas, V.; Koruza, J. Electromechanical properties of Ce-doped (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 lead-free piezoceramics. J. Adv. Ceram. 2019, 8, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Cai, W.; Li, Q.; Gao, R.; Chen, G.; Deng, X.; Wang, Z.; Cao, X.; Fu, C. Enhanced piezoelectric response of (Ba,Ca)(Ti,Zr)O3 ceramics by super large grain size and construction of phase boundary. J. Alloy. Compd. 2019, 794, 542–552. [Google Scholar] [CrossRef]
- Acosta, M.; Novak, N.; Rojas, V.; Patel, S.; Vaish, R.; Koruza, J.; Rossetti, G.A.; Rödel, J. BaTiO3-based piezoelectrics: Fundamentals, current status, and perspectives. Appl. Phys. Rev. 2017, 4, 041305. [Google Scholar] [CrossRef]
- Waqar, M.; Wu, H.; Chen, J.; Yao, K.; Wang, J. Evolution from lead-based to lead-free Piezoelectrics: Engineering of lattices, domains, boundaries, and defects leading to giant response. Adv. Mater. 2022, 34, 2106845. [Google Scholar] [CrossRef]
- Sluka, T.; Tagantsev, A.K.; Damjanovic, D.; Gureev, M.; Setter, N. Enhanced electromechanical response of ferroelectrics due to charged domain walls. Nat. Commun. 2012, 3, 748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Toward strong and tough glass and ceramic scaffolds for bone repair. Adv. Funct. Mater. 2013, 23, 5461–5476. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, L.C.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone mechanical properties in healthy and diseased states. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Zeng, Z.-Y.; Zhao, Y.-Q.; Lu, Q.; Cheng, Y. First-principles study of lattice dynamics, structural phase transition, and thermodynamic properties of barium titanate. Z. Für Naturforsch. A 2016, 71, 759–768. [Google Scholar] [CrossRef]
- Rho, J.-Y.; Tsui, T.Y.; Pharr, G.M. Elastic properties of human cortical and trabecular lamellar bone measured by nanoindentation. Biomaterials 1997, 18, 1325–1330. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Hodgson, P.; Wen, C. Elastic modulus and hardness of cortical and trabecular bovine bone measured by nanoindentation. Trans. Nonferrous Met. Soc. China 2006, 16, s744–s748. [Google Scholar] [CrossRef]
- Behrens, B.-A.; Wirth, C.J.; Windhagen, H.; Nolte, I.; Meyer-Lindenberg, A.; Bouguecha, A. Numerical investigations of stress shielding in total hip prostheses. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 593–600. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Gregory, T.; Hansen, U.; Cheng, C.-K. Effect of stress-shielding-induced bone resorption on glenoid loosening in reverse total shoulder arthroplasty. J. Orthop. Res. 2020, 38, 1566–1574. [Google Scholar] [CrossRef]
- Liverani, E.; Rogati, G.; Pagani, S.; Brogini, S.; Fortunato, A.; Caravaggi, P. Mechanical interaction between additive-manufactured metal lattice structures and bone in compression: Implications for stress shielding of orthopaedic implants. J. Mech. Behav. Biomed. Mater. 2021, 121, 104608. [Google Scholar] [CrossRef]
- Rana, M.; Chaudhuri, A.; Biswas, J.K.; Karim, S.I.; Datta, P.; Karmakar, S.K.; Roychowdhury, A. Design of patient specific bone stiffness mimicking scaffold. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2021, 235, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Sencadas, V.; Correia, D.M.; Lanceros-Méndez, S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf. B Biointerfaces 2015, 136, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, T.; Yu, Y.; Pang, Y.; Zhang, D.; Wang, Y.; Zhao, H.; Zhang, X.; Leng, H.; Yang, X.; Cai, Q. Improving bone regeneration with composites consisting of piezoelectric poly(l-lactide) and piezoelectric calcium/manganese co-doped barium titanate nanofibers. Compos. Part B Eng. 2022, 234, 109734. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A. Piezoelectric polymeric scaffold materials as biomechanical cellular stimuli to enhance tissue regeneration. Mater. Today Commun. 2022, 31, 103491. [Google Scholar] [CrossRef]
- Yang, C.; Ji, J.; Lv, Y.; Li, Z.; Luo, D. Application of piezoelectric material and devices in bone regeneration. Nanomaterials 2022, 12, 4386. [Google Scholar] [CrossRef]
- Damaraju, S.M.; Wu, S.; Jaffe, M.; Arinzeh, T.L. Structural changes in PVDF fibers due to electrospinning and its effect on biological function. Biomed. Mater. 2013, 8, 045007. [Google Scholar] [CrossRef]
- Teixeira, L.N.; Crippa, G.E.; Gimenes, R.; Zaghete, M.A.; de Oliveira, P.T.; Rosa, A.L.; Beloti, M.M. Response of human alveolar bone-derived cells to a novel poly(vinylidene fluoride-trifluoroethylene)/barium titanate membrane. J. Mater. Sci. Mater. Med. 2011, 22, 151–158. [Google Scholar] [CrossRef]
- Panda, A.K.; Sitaramgupta, V.S.N.; Pandya, H.J.; Basu, B. Electrical stimulation waveform-dependent osteogenesis on PVDF/BaTiO3 composite using a customized and programmable cell stimulator. Biotechnol. Bioeng. 2022, 119, 1578–1597. [Google Scholar] [CrossRef]
- Teixeira, L.N.; Crippa, G.E.; Trabuco, A.C.; Gimenes, R.; Zaghete, M.A.; Palioto, D.B.; de Oliveira, P.T.; Rosa, A.L.; Beloti, M.M. In vitro biocompatibility of poly(vinylidene fluoride–trifluoroethylene)/barium titanate composite using cultures of human periodontal ligament fibroblasts and keratinocytes. Acta Biomater. 2010, 6, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Lopes, H.B.; Santos, T.d.S.; de Oliveira, F.S.; Freitas, G.P.; de Almeida, A.L.; Gimenes, R.; Rosa, A.L.; Beloti, M.M. Poly(vinylidene-trifluoroethylene)/barium titanate composite for in vivo support of bone formation. J. Biomater. Appl. 2014, 29, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, R.; Zaghete, M.A.; Bertolini, M.; Varela, J.A.; Coelho, L.O.; Silva, N.F. Composites PVDF-TrFE/BT used as bioactive membranes for enhancing bone regeneration. In Proceedings of the Smart Structures and Materials 2004: Electroactive Polymer Actuators and Devices (EAPAD); Bar-Cohen, Y., Ed.; SPIE: Cergy, France, 2004; Volume 5385, p. 539. [Google Scholar] [CrossRef]
- dos Santos, G.G.; Malherbi, M.S.; de Souza, N.S.; César, G.B.; Tominaga, T.T.; Miyahara, R.Y.; de Mendonça, P.d.S.B.; Faria, D.R.; Rosso, J.M.; Freitas, V.F.; et al. 4th generation biomaterials based on PVDF-hydroxyapatite composites produced by electrospinning: Processing and characterization. Polymers 2022, 14, 4190. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Ghaee, A.; Barzin, J. Preparation and characterization of a piezoelectric poly (vinylidene fluoride)/nanohydroxyapatite scaffold capable of naproxen delivery. Eur. Polym. J. 2019, 112, 442–451. [Google Scholar] [CrossRef]
- Rodrigues, P.J.G.; Elias, C.d.M.V.; Viana, B.C.; de Hollanda, L.M.; Stocco, T.D.; de Vasconcellos, L.M.R.; Mello, D.d.C.R.; Santos, F.E.P.; Marciano, F.R.; Lobo, A.O. Electrodeposition of bactericidal and bioactive nano-hydroxyapatite onto electrospun piezoelectric polyvinylidene fluoride scaffolds. J. Mater. Res. 2020, 35, 3265–3275. [Google Scholar] [CrossRef]
- Malherbi, M.S.; Dias, L.C.; Lima, M.S.Z.; Ribeiro, L.G.; Freitas, V.F.; Bonadio, T.G.M.; Silva, L.M.; Souza, G.B.; Volnistem, E.A.; Rosso, J.M.; et al. Electrically stimulated bioactivity in hydroxyapatite/β-tricalcium phosphate/polyvinylidene fluoride biocomposites. J. Mater. Res. Technol. 2022, 20, 169–179. [Google Scholar] [CrossRef]
- Ribeiro, C.; Correia, D.M.; Rodrigues, I.; Guardão, L.; Guimarães, S.; Soares, R.; Lanceros-Méndez, S. In vivo demonstration of the suitability of piezoelectric stimuli for bone reparation. Mater. Lett. 2017, 209, 118–121. [Google Scholar] [CrossRef]
- Reis, J.; Frias, C.; Canto e Castro, C.; Botelho, M.L.; Marques, A.T.; Simões, J.A.O.; Capela e Silva, F.; Potes, J. A new piezoelectric actuator induces bone formation in vivo: A preliminary study. J. Biomed. Biotechnol. 2012, 2012, 613403. [Google Scholar] [CrossRef]











| CERAMIC DISKS DENSIFIED THROUGH CONVENTIONAL SINTERING PROCESS | |||||
|---|---|---|---|---|---|
| Material | Calcination Temperature (°C) | Pressing Force (kg/cm2) | Atmosphere | Sintering Temperature (°C) | Sintering Duration (h) |
| BT | 1000 | 200 | Air | 1300 | 3 |
| Zr:BT | 1000 | 150 | Air | 1300 | 3 |
| BCTZ50 | 1350 | 80 | Air | 1550 | 4 |
| LNO | 800 | 80 | Air | 1175 * | 2 |
| KNO | 700 | 50 | Air | 1000 * | 2 |
| LTO | 700 | 40 | Air | 1375 | 3 |
| CERAMIC DISKS DENSIFIED THROUGH SPARK PLASMA SINTERING (SPS) | |||||
| Material | Calcination temperature(°C) | Pressing force (MPa) | Atmosphere | Sintering temperature(°C) | Sintering duration (min) |
| KNO | 600 | 60 | Vacuum, 40 hPa | 900 | 3 |
| LTO | 800 | 60 | Vacuum, 40 hPa | 1150 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedelcu, L.; Ferreira, J.M.F.; Popa, A.-C.; Amarande, L.; Nan, B.; Bălescu, L.-M.; Geambașu, C.D.; Cioangher, M.-C.; Leonat, L.; Grigoroscuță, M.; et al. Multi-Parametric Exploration of a Selection of Piezoceramic Materials for Bone Graft Substitute Applications. Materials 2023, 16, 901. https://doi.org/10.3390/ma16030901
Nedelcu L, Ferreira JMF, Popa A-C, Amarande L, Nan B, Bălescu L-M, Geambașu CD, Cioangher M-C, Leonat L, Grigoroscuță M, et al. Multi-Parametric Exploration of a Selection of Piezoceramic Materials for Bone Graft Substitute Applications. Materials. 2023; 16(3):901. https://doi.org/10.3390/ma16030901
Chicago/Turabian StyleNedelcu, Liviu, José M. F. Ferreira, Adrian-Claudiu Popa, Luminița Amarande, Bo Nan, Liliana-Marinela Bălescu, Cezar Dragoș Geambașu, Marius-Cristian Cioangher, Lucia Leonat, Mihai Grigoroscuță, and et al. 2023. "Multi-Parametric Exploration of a Selection of Piezoceramic Materials for Bone Graft Substitute Applications" Materials 16, no. 3: 901. https://doi.org/10.3390/ma16030901