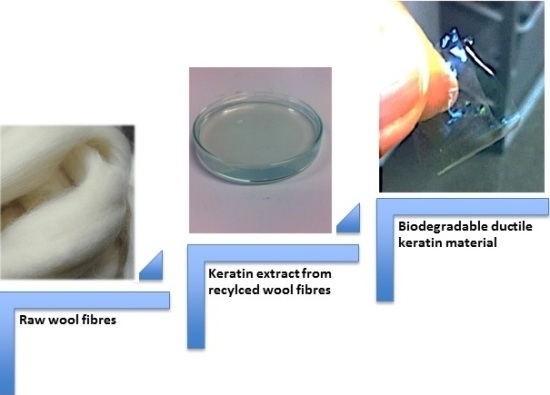
Reconstituted Keratin Biomaterial with Enhanced Ductility
Abstract
:1. Introduction
2. Results and Discussion
2.1. Keratin Extraction, Purification and Characterization



2.2. Keratin Film Characterization



2.2.1. Mechanical Properties of the Keratin Film
| Keratin Film | Ultimate Strength (MPa) | Young Modulus (MPa) | Elongation (%) |
|---|---|---|---|
| Compression molded [35] | 20–27 | 710–1218 | 1–4 |
| Non-treated keratin film [38] | - | - | - |
| Chitosan/keratin film [38] | 9–14 | 92–111 | 18–31 |
| Chitosan/Glycerol/keratin [38] | 27–34 | 160–310 | 4–5 |
| Dry keratin film for ocular surface regeneration [12] | 20 | 300 | - |
| Dry Keratin film from this study | 20–30 | 12–15 | 30–40 |

2.2.2. Cell Culture Experiments


3. Experimental Section

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Poole, A.J.; Church, J.S.; Huson, M.G. Environmentally sustainable fibers from regenerated protein. Biomacromolecules 2008, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ottoman, J.A.; Stafford, E.R.; Hartman, C.L. Avoiding green marketing myopia: Ways to improve consumer appeal for environmentally preferable products. Environ. Sci. Policy Sustain. Dev. 2006, 48, 22–36. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable bio-composites from renewable resources: Opportunities and challenges in the green materials world. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Guille, M.M.G.; Mosser, G.; Helary, C.; Eglin, D. Bone matrix like assemblies of collagen: From liquid crystals to gels and biomimetic materials. Micron 2005, 36, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.R.; Eaton, J.W. Albumin binding surfaces for biomaterials. J. Lab. Clin. Med. 1994, 124, 537–545. [Google Scholar] [PubMed]
- Sivakumar, M.; Rao, K.P. Preparation, characterization and in vitro release of gentamicin from coralline hydroxyapatite–gelatin composite microspheres. Biomaterials 2002, 23, 3175–3181. [Google Scholar] [CrossRef]
- Kang, H.W.; Tabata, Y.; Ikada, Y. Fabrication of porous gelatin scaffolds for tissue engineering. Biomaterials 1999, 20, 1339–1344. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Omenetto, F.G.; Kaplan, D. A new route for silk. Nat. Photonics 2008, 2, 641–643. [Google Scholar] [CrossRef]
- Rouse, J.G.; van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef]
- Aluigi, A.; Vineis, C.; Ceria, A.; Tonin, C. Composite biomaterials from fiber wastes: Characterization of wool–cellulose acetate blends. Compos. Part A Appl. Sci. Manuf. 2008, 39, 126–132. [Google Scholar] [CrossRef]
- Reichl, S.; Borrelli, M.; Geerling, G. Keratin films for ocular surface reconstruction. Biomaterials 2011, 32, 3375–3386. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, T. Protein fibers as performance proteins: New technologies and applications. Curr. Opin. Biotech. 2005, 16, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Strelkov, S.V.; Herrmann, H.; Aebi, U. Molecular architecture of intermediate filaments. Bioassays 2003, 25, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Salminen, E.; Rintala, J. Anaerobic digestion of organic solid poultry slaughterhouse waste—A review. Bioresour. Technol. 2002, 83, 13–26. [Google Scholar] [CrossRef]
- Schrooyen, P.M.M.; Dijkstra, P.J.; Oberthür, R.C.; Bantjes, A.; Feijen, J. Stabilization of solutions of feather keratins by sodium dodecyl sulfate. J. Colloid. Interf. Sci. 2001, 240, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Onifade, A.A.; Al-Sane, N.A.; Al-Musallam, A.A.; Al-Zarban, A. A review: Potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresour. Technol. 1998, 66, 1–11. [Google Scholar] [CrossRef]
- European Man Made Fiber Association. Available online: http://www.cirfs.org (accessed on 29 October 2015).
- Hearle, J.W.S.; Woodings, C. Fibers Related to Cellulose. In Regenerated Cellulose Fibers; Woodhead Publishing: Sawston, Cambridge, UK, 2001; pp. 156–173. [Google Scholar]
- Ministry of Textiles. Available online: http://www.texmin.nic.in/policy/Fibre_Policy_Sub_%20Groups (accessed on 26 October 2015).
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Padgett, T.; Han, I.; Dawson, P. Incorporation of food-grade antimicrobial compounds into biodegradable packaging films. J. Food Prot. 1998, 61, 1330–1335. [Google Scholar] [PubMed]
- Zahn, H.; Föhles, J.; Nlenhaus, M.; Schwan, A.; Spel, M. Wool as a biological composite structure. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 496–501. [Google Scholar] [CrossRef]
- Fudge, D.S.; Gardner, K.H.; Forsyth, V.T.; Riekel, C.; Gosline, J.M. The mechanical properties of hydrated intermediate filaments: Insights from hagfish slime threads. Biophys. J. 2003, 85, 2015–2027. [Google Scholar] [CrossRef]
- Bertaud, J.; Qin, Z.; Buehler, M.J. Intermediate filament-deficient cells are mechanically softer at large deformation: A multi-scale simulation study. Acta Biomater. 2010, 6, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.; O'donnell, I. Studies on oxidised wool. I. A comparison of the completeness of oxidation with peracetic and performic acid. Aust. J. Biol. Sci. 1959, 12, 282–293. [Google Scholar]
- Mchugh, T.H.; Weller, C.L.; Krochta, J.M. Edible Coatings and Films to Improve Food Quality; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Krochta, J.M. Proteins as Raw Materials for Films and Coatings. In Protein-Based Films and Coatings; Gennadios, A., Ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 1–41. [Google Scholar]
- Tachibana, A.; Furuta, Y.; Takeshima, H.; Tanabe, T.; Yamauchi, K. Fabrication of wool keratin sponge scaffolds for long-term cell cultivation. J. Biotech. 2002, 93, 165–170. [Google Scholar] [CrossRef]
- Katoh, K.; Tanabe, T.; Yamauchi, K. Novel approach to fabricate keratin sponge scaffolds with controlled pore size and porosity. Biomaterials 2004, 25, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, S.; Tachibana, A.; Tada, D.; Yamauchi, K.; Tanabe, T. Fabrication of highly porous keratin sponges by freeze-drying in the presence of calcium alginate beads. Mater. Sci. Eng. C 2008, 28, 1250–1254. [Google Scholar] [CrossRef]
- Aluigi, A.; Varesano, A.; Montarsolo, A.; Vineis, C.; Ferrero, F.; Mazzuchetti, G.; Tonin, C. Electrospinning of keratin/poly (ethylene oxide) blend nanofibers. J. Appl. Polym. Sci. 2007, 104, 863–870. [Google Scholar] [CrossRef]
- Zoccola, M.; Aluigi, A.; Vineis, C.; Tonin, C.; Ferrero, F.; Piacentino, M.G. Study on cast membranes and electrospun nanofibers made from keratin/fibroin blends. Biomacromolecules 2008, 9, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Yamauchi, A.; Kusunoki, T.; Kohda, A.; Konishi, Y. Preparation of stable aqueous solution of keratins, and physiochemical and biodegradational properties of films. J. Biomed. Mater. Res. 1998, 31, 439–444. [Google Scholar] [CrossRef]
- Katoh, K.; Shibayama, M.; Tanabe, T.; Yamauchi, K. Preparation and physicochemical properties of compression-molded keratin films. Biomaterials 2004, 25, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Howie, A.; Brewer, D.B.; Howell, D.; Jones, A.P. Physical basis of colors seen in Congo red-stained amyloid in polarized light. Lab. Invest. 2008, 88, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Vineis, C.; Varesano, A.; Mazzuchetti, G.; Ferrero, F.; Tonin, C. Structure and properties of keratin/PEO blend nanofibers. Euro. Pol. J. 2008, 44, 2465–2475. [Google Scholar] [CrossRef]
- Tanabe, T.; Okitsu, N.; Tachibana, A.; Yamauchi, K. Preparation and characterization of keratin–chitosan composite film. Biomaterials 2002, 23, 817–825. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Boil. Chem. 1951, 193, 265–275. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atri, H.; Bidram, E.; Dunstan, D.E. Reconstituted Keratin Biomaterial with Enhanced Ductility. Materials 2015, 8, 7472-7485. https://doi.org/10.3390/ma8115392
Atri H, Bidram E, Dunstan DE. Reconstituted Keratin Biomaterial with Enhanced Ductility. Materials. 2015; 8(11):7472-7485. https://doi.org/10.3390/ma8115392
Chicago/Turabian StyleAtri, Halleh, Elham Bidram, and David E. Dunstan. 2015. "Reconstituted Keratin Biomaterial with Enhanced Ductility" Materials 8, no. 11: 7472-7485. https://doi.org/10.3390/ma8115392