A 3D Coordination Polymer Based on Syn-Anti Bridged [Mn(RCOO)2]n Chains Showing Spin-Canting with High Coercivity and an Ordering Temperature of 14 K
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis
3.2. Structure of [Mn3(HL)6] (1)
3.3. Hirshfeld Surface Analysis
3.4. Absorption and Emission Spectra of [Mn3(HL)6] (1)
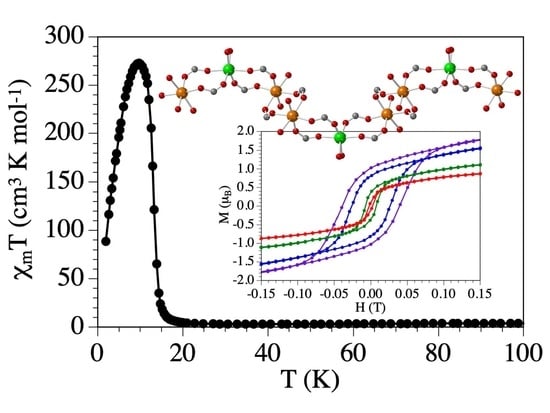
3.5. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakamaki, Y.; Tsuji, M.; Heidrick, Z.; Watson, O.; Durchman, J.; Salmon, C.; Burgin, S.R.; Beyzavi, H. Preparation and Applications of Metal−Organic Frameworks (MOFs): A Laboratory Activity and Demonstration for High School and/or Undergraduate Students. J. Chem. Educ. 2020, 97, 1109–1116. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.; Li, J.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Cheng, X.; Xie, Z.; Kuang, Q.; Zheng, L. The function of metal–organic frameworks in the application of MOF-based composites. Nanoscale Adv. 2020, 2, 2628. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Hmoudah, M.; Broccoli, F.; Iesce, M.R.; Jung, O.; Serio1, M.D. Applications of Metal Organic Frameworks in Wastewater Treatment: A Review on Adsorption and Photodegradation. Front. Chem. Eng. 2020, 2, 581487. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal-Organic Framework Magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Dixit, R.; Sharma, S.; Dutta, S.; Solanki, K.; Sharma, R.K. Magnetic metal–organic framework composites: Structurally advanced catalytic materials for organic transformations. Mater. Adv. 2021, 2, 2153–2187. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, G.; Gao, M.; Huang, X.; Xu, D. Recent Advances and Applications of Magnetic Metal-Organic Frameworks in Adsorption and Enrichment Removal of Food and Environmental Pollutants. Crit. Rev. Anal. Chem. 2020, 50, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Kurmoo, M. Magnetic metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Espallargas, G.M. Dynamic magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525. [Google Scholar] [CrossRef]
- Ricco, R.; Malfatti, L.; Takahashi, M.; Hill, A.J.; Falcaro, P. Applications of magnetic metal–organic framework composites. J. Mater. Chem. A 2013, 1, 13033. [Google Scholar] [CrossRef]
- Ammari, Y.; Baaalla, N.; Hlil, E.K.; Abid, S. Structure, optical and magnetic properties of a novel homometallic coordination polymers: Experimental and computational studies. Nat. Sci. Rep. 2020, 10, 1316. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.; Leong, C.F.; D’Alessandro, D.M.; Zuo, J. Crystal Structures, Magnetic Properties, and Electrochemical Properties of Coordination Polymers Based on the Tetra(4-pyridyl)- tetrathiafulvalene Ligand. Inorg. Chem. 2015, 54, 10766–10775. [Google Scholar] [CrossRef]
- Shao, D.; Moorthy, S.; Yang, X.; Yang, J.; Shi, L.; Singh, S.K.; Tian, Z. Tuning the structure and magnetic properties via distinct pyridine derivatives in cobalt(II) coordination polymers. Dalton Trans. 2022, 51, 695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, L.; Zhou, W.; Wei, X.; Hu, T. Synthesis and magnetic properties of two Mn based coordination polymers constructed by a mixed-ligand strategy. CrystEngComm 2020, 22, 7123. [Google Scholar] [CrossRef]
- Pajuelo-Corral, O.; García, J.A.; Castillo, O.; Luque, A.; Rodríguez-Diéguez, A.; Cepeda, J. Single-Ion Magnet and Photoluminescence Properties of Lanthanide(III) Coordination Polymers Based on Pyrimidine-4,6-Dicarboxylate. Magnetochemistry 2021, 7, 8. [Google Scholar] [CrossRef]
- Zhou, Y.; Hong, M.; Wu, X. Lanthanide–transition metal coordination polymers based on multiple N- and O-donor ligands. Chem. Commun. 2006, 2, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, L.; Wang, L.; Song, H.; Qiang, Z.; Wu, S.X.; Ng, S. Lanthanide coordination polymers based on multi-donor ligand containing pyridine and phthalate moieties: Structures, luminescence and magnetic properties. J. Solid State Chem. 2013, 206, 277–285. [Google Scholar] [CrossRef]
- Robin, A.Y.; Fromm, K.M. Coordination polymer networks with O- and N-donors: What they are, why and how they are made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Galán-Mascarós, J.R.; Dunbar, K.R. A Self-Assembled 2D Molecule-Based Magnet: The Honeycomb Layered Material {Co3Cl4(H2O)2[Co(Hbbiz)3]2]. Angew. Chem. Int. Ed. 2003, 42, 2289. [Google Scholar] [CrossRef]
- Maspoch, D.; Ruiz-Molina, D.; Veciana, J. Magnetic nanoporous coordination polymers. J. Mater. Chem. 2004, 14, 2713. [Google Scholar] [CrossRef]
- Poulsen, R.D.; Bentien, A.; Chevalier, M.; Iversen, B.B. Synthesis, Physical Properties, Multitemperature Crystal Structure, and 20 K Synchrotron X-ray Charge Density of a Magnetic Metal Organic Framework Structure, Mn3(C8O4H4)3(C5H11ON)2. J. Am. Chem. Soc. 2005, 127, 9156. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, H.; Clérac, R.; Mizushima, K.; Sugiura, K.I.; Yamashita, M.; Wernsdorfer, W.; Coulon, C. [Mn2(saltmen)2Ni(pao)2(L)2](A)2 with L = Pyridine, 4-Picoline, 4-tert-Butylpyridine, N-Methylimidazole and A = ClO4−, BF4−, PF6−, ReO4−: A Family of Single-Chain Magnets. Inorg. Chem. 2003, 42, 8203–8213. [Google Scholar] [CrossRef] [PubMed]
- Clérac, R.; Miyasaka, H.; Yamashita, M.; Coulon, C. Evidence for Single-Chain Magnet Behavior in a MnIII−NiII Chain Designed with High Spin Magnetic Units: A Route to High Temperature Metastable Magnets. J. Am. Chem. Soc. 2002, 124, 12837–12844. [Google Scholar] [CrossRef]
- Kimura, S.; Matsuoka, R.; Kimura, S.; Nishihara, H.; Kusamoto, T. Radical-Based Coordination Polymers as a Platform for Magnetoluminescence. J. Am. Chem. Soc. 2021, 143, 5610–5615. [Google Scholar] [CrossRef]
- Yong, G.; Qiao, S.; Wang, Z. A One-Dimensional Coordination Polymer Based on Novel Radical Anion Ligand Generated In Situ: Notable Magnetic and Luminescence Properties. Cryst. Growth Des. 2008, 8, 1465–1467. [Google Scholar] [CrossRef]
- Benmansour, S.; Abherve, A.; Gómez-Claramunt, P.; Vallés-García, C.; Gómez-García, C.J. Nanosheets of Two-Dimensional Magnetic and Conducting Fe(II)/Fe(III) Mixed-Valence Metal−Organic Frameworks. ACS Appl. Mater. Interfaces 2017, 9, 26210–26218. [Google Scholar] [CrossRef]
- Aulakh, D.; Liu, L.; Varghese, J.R.; Xie, H.; Islamoglu, T.; Duell, K.; Kung, C.; Hsiung, C.; Zhang, Y.; Drout, R.J.; et al. Direct Imaging of Isolated Single-Molecule Magnets in Metal-Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 2997–3005. [Google Scholar] [CrossRef]
- Palacios-Corella, M.; García-López, V.; Sánchez-Sánchez, C.; Clemente-Juan, J.M.; Clemente-León, M.; Coronado, E. Insertion of single-ion magnets based on mononuclear Co(II) complexes into ferromagnetic oxalate-based networks. Dalton Trans. 2021, 50, 5931. [Google Scholar] [CrossRef]
- Kusumoto, S.; Umeno, H.; Kim, Y.; Sekine, Y.; Nakamura, M.; Hayami, S. Structural and Magnetic Characterization of Homo- and Heterometallic Trinuclear Ni(II) and Cu(II) Clusters with N2O6 Acyclic Polydentate Ligand. Chem. Lett. 2021, 50, 1945–1948. [Google Scholar] [CrossRef]
- Andrews, P.C.; Deacon, G.B.; Frank, R.; Fraser, B.H.; Junk, P.C.; MacLellan, J.G.; Massi, M.; Moubaraki, B.; Murray, K.S.; Silberstein, M. Formation of HoIII Trinuclear Clusters and GdIII Monodimensional Polymers Induced by ortho and para Regioisomers of Pyridyl-Functionalised β-Diketones: Synthesis, Structure, and Magnetic Properties. Eur. J. Inorg. Chem. 2009, 2009, 744–751. [Google Scholar] [CrossRef]
- Gao, E.; Liu, N.; Cheng, A.; Gao, S. Novel frustrated magnetic lattice based on triangular [Mn3(μ3-F)] clusters with tetrazole ligands. Chem. Commun. 2007, 24, 2470–2472. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Ohashi, Y. 2-Oxo- 1,2-dihydropyridine-6-carboxylic Acid. Acta Cryst. 1998, C54, 1491–1493. [Google Scholar] [CrossRef]
- Kazemi, S.H.; Eshtiagh-Hosseini, H.; Izadyar, M.; Mirzaei, M. Computational Study of the Intramolecular Proton Transfer between 6- Hydroxypicolinic Acid Tautomeric Forms and Intermolecular Hydrogen Bonding in their Dimers. Phys. Chem. Res. 2013, 1, 117–125. [Google Scholar]
- Liu, C.; Song, Y. An Uneven Chain-like Ferromagnetic Copper(II) Coordination Polymer Displaying Metamagnetic Behavior and Long-Range Magnetic Ordering. Magnetochemistry 2022, 8, 2. [Google Scholar] [CrossRef]
- Sun, C.; Zheng, X.; Li, W.; Wang, M.; Fang, C. Assembly of Supramolecular Networks with the Inclusion of Water Chains, Cyclic Hepta and Octa Water Clusters. Z. Anorg. Allg. Chem. 2008, 634, 26632669. [Google Scholar] [CrossRef]
- Bian, G.Q.; Kuroda-Sowa, T.; Konaka, H.; Maekawa, M.; Munakata, M. Bis(μ-6-hydroxypicolinato)-μ-oxobis[dipyridinemanganese (III)] monohydrate. Acta Crystallogr. Sect. C 2004, 60, m338–m340. [Google Scholar] [CrossRef]
- Sun, C.; Zheng, X.; Jin, L.Z. Syntheses and Structures of the First Examples of Lanthanide Complexes with 6-Hydroxypicolinic Acid. Z. Anorg. Allg. Chem. 2004, 630, 13421347. [Google Scholar] [CrossRef]
- Kukovec, B.; Vaz, P.D.; Calhorda, M.J.; Popovic, Z. Disappearing and Concomitant Polymorphism of Nickel(II) Complexes with 6-Hydroxypicolinic Acid. Structural and Density Functional Theory Studies. Cryst. Growth Des. 2010, 10, 3685–3693. [Google Scholar] [CrossRef]
- Bruker, APEX2, SAINT and SADABS, BRUKER AXS, Inc.: Madison, WI, USA, 2008.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows, an update. J. Appl. Crystallogr. 2012, 45, 849. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELX. Acta Cryst. 2015, C71, 3. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Kukovec, B.; Popovića, Z.; Pavlović, G.; Linarić, M.R. Synthesis and structure of cobalt(II) complexes with hydroxyl derivatives of pyridinecarboxylic acids: Conformation analysis of ligands in the solid state. J. Mol. Struct. 2008, 882, 47–55. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Wang, X.; Wei, H.; Wang, Z.; Chen, Z.; Gao, S. Formate—The Analogue of Azide: Structural and Magnetic Properties of M(HCOO)2(4,4‘-Bpy)·nH2O (M = Mn, Co, Ni; n = 0, 5). Inorg. Chem. 2005, 44, 572–583. [Google Scholar] [CrossRef]
- Kar, P.; Guha, P.M.; Drew, M.G.B.; Ishida, T.; Ghosh, A. Spin-Canted Antiferromagnetic Phase Transitions in Alternating Phenoxo- and Carboxylato-Bridged MnIII-Salen Complexes. Eur. J. Inorg. Chem. 2011, 2011, 2075–2085. [Google Scholar] [CrossRef]
- Mossin, S.; Weihe, H.; Osholm Sørensen, H.; Lima, N.; Sessoli, R. Rationalisation of Weak Ferromagnetism in Manganese(iii) Chains: The Relation between Structure and Ordering Phenomena. Dalton Trans. 2004, 4, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Molecular Magnetism; VCH Publishers: New York, NY, USA, 1993. [Google Scholar]
- Kang, S.K.; Shim, Y.S. Poly[(m6-6-Oxidopyridinium-2-Carboxylato)Caesium]. Acta Cryst. E 2011, 67, m1237. [Google Scholar] [CrossRef]
- Małecki, J.G.; Krompiec, S.; Maroń, A.; Penkala, M. Synthesis, Molecular, Spectroscopic and Catalytic Characterization of Ruthenium(II) Complexes with Pyridine-2-Carboxylic Acid Derivatives Ligands. Polyhedron 2012, 48, 21–30. [Google Scholar] [CrossRef]
- Kukovec, B.-M.; Popovic, Z.; Pavlovic, G. Copper(II) Complexes with 3- and 6-hydroxypicolinic Acid. Preparation, Structural, Spectroscopic and Thermal Study. Acta Chim. Slov. 2008, 55, 779–787. [Google Scholar]
- Kukovec, B.M.; Kaksa, M.; Popovic, Z. Synthesis and Characterization of a Copper(II) Complex with 6-Hydroxypicolinic Acid and 3-Picoline Croatica. Chem. Acta 2012, 85, 479–483. [Google Scholar]
- Sengül, A.; Büyükgüngör, O. Trans-Di-Aqua-Bis(6-Hydroxy-Picolinato-k2-N,O2)Copper(II). Acta Cryst. E 2005, 61, m119–m121. [Google Scholar]
- You-Zhu Yu, Song-Yang Chang, Xi Han, Guang-Xin Chen, Ya-Wei Xuan, Xian-Li Wu, Fang Wang, Hydrothermal Syntheses, Crystal Structures and Luminescence Properties of Cu(II) and Cd(II) Complexes Assembled by 6-Hydroxypicolinic Acid and 1,10-Phenanthroline. Jiegou Huaxue 2019, 38, 651–659.
- Sun, C.; Zhou, J.; Li, W.; Jin, L. Supramolecular Stuctures from Mononuclear, Binuclear and 2D Net of Copper(II) Complexes with 6-Hydroxypicolinic Acid. Z. Anorg. Allg. Chem. 2008, 634, 549–554. [Google Scholar] [CrossRef]
- Su, W.; Guo, Y.; Yu, Y.; Wang, Y. Synthesis, Structure and Magnetic Property of a Linear Trinuclear Dy(III) Single Molecular Magnet. Inorg. Chem. Commun. 2020, 120, 108161. [Google Scholar] [CrossRef]
- Nakasone, T.; Nishioka, T.; Asato, E.; Kinoshita, I.; Takara, S. Synthesis and Structural Characterization of Novel Ruthenium(II) Complexes Bearing Hydroxypicolinato Ligands. Polyhedron 2012, 45, 152–157. [Google Scholar] [CrossRef]
- Casas, J.S.; Castellano, E.E.; Ellena, J.; García-Tasende, M.S.; Sánchez, A.; Sordo, J.; Toma, M. Dimethylthallium(III) Complexes with Picolinic Acid and its Hydroxyl Derivatives. Polyhedron 2008, 27, 1296–1302. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Fanwick, P.E.; Walton, R.A. Reactions of the Triply-Bonded Complex cis-Re2(μ-O2CCH3)2Cl2(μ-Dppm)2 with Pyridine Carboxylic Acids. the Isolation and Structural Characterization of a Third Structural Isomer of Re2(Dipic)Cl2(μ-Dppm)2 (Dipic = Pyridine-2,6-Dicarboxylate). Dalton Trans. 2003, 18, 3617–3621. [Google Scholar] [CrossRef]
- Mukiza, J.; Hosten, E.C.; Gerber, T.I.A. Rhenium(III), (IV) and (V) Complexes with 6-Hydroxypicolinic Acid. Polyhedron 2016, 110, 106–113. [Google Scholar] [CrossRef]
- Nakama, Y.; Nishioka, T.; Nakasone, T.; Asato, E.; Kinoshita, I.; Takara, S. Synthesis And Crystal Structure of [RuCl(6-hydroxypicolinato)(2,2’;6’,2”-terpyridine)]·(N,N-dimethylformamide). X-ray Struct. Anal. Online 2010, 26, 33–34. [Google Scholar] [CrossRef]
- Sun, C.; Jin, L. Supramolecular Architectures from the Self-Assembly of Lanthanide Ions with 6-Hydroxypicolinic Acid and 1,10-Phenanthroline. J. Mol. Struct. 2005, 741, 241–247. [Google Scholar] [CrossRef]









| Formula | C36H24Mn3N6O18 |
|---|---|
| Formula weight | 993.43 |
| Crystal system | Monoclinic |
| Space group | C2/c (No. 15) |
| a (Å) | 12.3872 (13) |
| b (Å) | 15.4999 (13) |
| c (Å) | 20.7172 (17) |
| α (°) | 90 |
| β (°) | 105.713 (4) |
| γ (°) | 90 |
| V (Å3) | 3829.1 (6) |
| Z | 4 |
| ρcalc (g/cm3) | 1.723 |
| μ (Mo Kα) (mm) | 1.065 |
| F(000) | 2004 |
| Crystal size (mm3) | 0.10 × 0.12 × 0.16 |
| Temperature, T (K) | 127 |
| θmin-max (deg) | 2.6, 27.2 |
| Total data | 21,514 |
| Unique data | 4249 |
| Rint | 0.079 |
| Observed data [I > 2.0 σ(I)] | 3104 |
| Nref | 4249 |
| Npar | 294 |
| R | 0.0479 |
| wR2 | 0.1177 |
| S | 1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubey, S.K.; Patra, M.; Gupta, K.; Bhattacharjee, S.; Saha, R.; Gómez-García, C.J. A 3D Coordination Polymer Based on Syn-Anti Bridged [Mn(RCOO)2]n Chains Showing Spin-Canting with High Coercivity and an Ordering Temperature of 14 K. Magnetochemistry 2023, 9, 55. https://doi.org/10.3390/magnetochemistry9020055
Dubey SK, Patra M, Gupta K, Bhattacharjee S, Saha R, Gómez-García CJ. A 3D Coordination Polymer Based on Syn-Anti Bridged [Mn(RCOO)2]n Chains Showing Spin-Canting with High Coercivity and an Ordering Temperature of 14 K. Magnetochemistry. 2023; 9(2):55. https://doi.org/10.3390/magnetochemistry9020055
Chicago/Turabian StyleDubey, Soumen Kumar, Maxcimilan Patra, Kajal Gupta, Subham Bhattacharjee, Rajat Saha, and Carlos J. Gómez-García. 2023. "A 3D Coordination Polymer Based on Syn-Anti Bridged [Mn(RCOO)2]n Chains Showing Spin-Canting with High Coercivity and an Ordering Temperature of 14 K" Magnetochemistry 9, no. 2: 55. https://doi.org/10.3390/magnetochemistry9020055