New Isocoumarin Derivatives and Meroterpenoids from the Marine Sponge-Associated Fungus Aspergillus similanensis sp. nov. KUFA 0013
Abstract
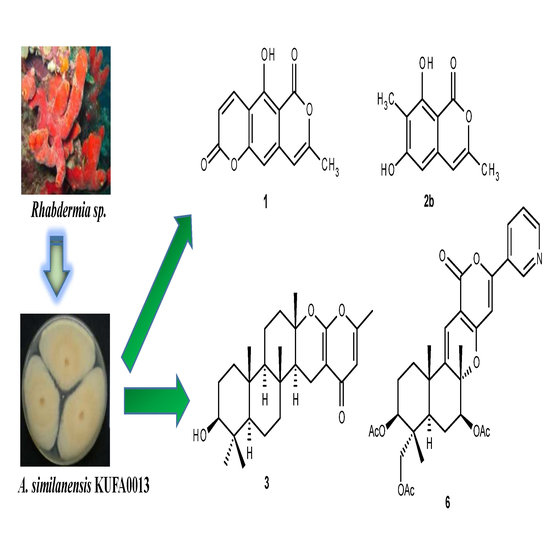
:1. Introduction

2. Results and Discussion
| Position | δC, Type | δH, (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 2 | 159.7, C | - | ||
| 3 | 114.1, CH | 6.36, d (9.8) | H-4 | 10a |
| 4 | 137.8, CH | 8.13, d (9.8) | H-3 | C-2, 4a |
| 4a | 107.3, C | - | ||
| 5 | 160.3, C | - | ||
| 5a | 101.3, C | - | ||
| 6 | 166.3, C | - | ||
| 8 | 156.2, C | - | ||
| 9 | 104.6, CH | 6.33, s | CH3-8 | C-5a, 8, 10, Me-8 |
| 9a | 130.0, C | - | ||
| 10 | 102.7, CH | 6.70, s | C-4a, 5a, 9a, 10a | |
| 10a | 140.1, C | - | ||
| CH3-8 | 19.6, CH3 | 2.33, s | H-9 | C-8, 9 |
| OH-5 | - | 11.90, s | C-4a, 5, 5a |
| Position | δC, Type | δH, (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 | 166.1, CO | - | ||
| 3 | 153.3, C | - | ||
| 4 | 104.2, CH | 6.46, s | CH3-3 | C-5, 8a |
| 4a | 136.5, C | - | ||
| 5 | 101.4, CH | 6.40, s | C-4, 6, 7, 8a | |
| 6 | 163.7, C | - | ||
| 7 | 109.6, C | - | ||
| 8 | 160.0, C | - | ||
| 8a | 97.5, C | - | ||
| CH3-3 | 18.8, CH3 | 2.20, s | C-3, 4 | |
| CH3-7 | 8.0, CH3 | 2.00, s | C-6, 7, 8 | |
| OH-6 | - | 3.45, br | ||
| OH-8 | - | 11.27, s | C-7, 8, 8a |

| Position | δC, Type | δH, (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 | 73.2, CH | 4.79, dd (11.7, 4.6) | H-2 | |
| 2 | 23.2, CH2 | 1.99, m | H-1 | |
| 1.76, m | ||||
| 3 | 35.5, CH2 | 2.09, m | H-2 | |
| 4 | 38.8, C | - | ||
| 5 | 144.5, C | - | ||
| 6 | 83.9, C | - | ||
| 7 | 77.7, CH | 5.23, dd (11.9, 5.4) | H-8 | C-6 |
| 8 | 24.3, CH2 | 1.82, ddd (12.8, 5.1, 1.4) | H-7, H-9 | |
| 1.61, m | ||||
| 9 | 41.1, CH | 1.73, brd (12.5) | H-8 | |
| 10 | 40.6, C | - | ||
| 11 | 64.7, CH2 | 3.75, d (11.9) | C-1, 9, CO (OAc-11) | |
| 3.79, d (11.9) | ||||
| 12 | 24.2, CH3 | 1.26, s | C-3, 4, 9 | |
| 13 | 111.2, CH | 6.36, s | C-4, 6, 4′ | |
| 14 | 21.3, CH3 | 1.59,s | C-5, 6, 7 | |
| 15 | 13.3, CH3 | 0.88, s | C-1, 10, 11 | |
| 2′ | 161.3, C | - | ||
| 3′ | 101.1, C | - | ||
| 4′ | 161.2, C | - | ||
| 5′ | 98.6, CH | 6.54, s | C-2′, 3′, 6′, 3″ | |
| 6′ | 157.2, C | - | ||
| 2″ | 146.6, CH | 9.02, brs | H-4″ | C-3″, 4″, 6″ |
| 3″ | 127.4, C | - | ||
| 4″ | 133.1, CH | 8.14, dt (7.8, 1.4, 1.4) | H-2″, 5″ | C-6′, 2″, 6″ |
| 5″ | 123.8, CH | 7.42, dd (8.0, 4.9) | H-4″, 6″ | C-3″, 6″ |
| 6″ | 151.2, CH | 8.68, brd (4.0) | H-5″ | C-5″ |
| OAc-1 | 170.4, CO | - | ||
| 21.2, CH3 | 2.05, s | CO (OAc-1) | ||
| OAc-7 | 169.8, CO | - | ||
| 21.2, CH3 | 2.17, s | CO (OAc-7) | ||
| OAc-11 | 171.0, CO | - | ||
| 20.8, CH3 | 2.10, s | CO (OAc-11) |
| Compound | E. coli G1 | S. aureus B1 | E. faecalis W1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotics | ||||||||||
| CIP | AMP | CTX | S | OX | AMP | CIP | VA | AMP | E | |
| 1 | − | − | − | − | − | − | − | − | − | − |
| 2a | − | − | − | − | − | ++ | − | − | − | − |
| 2b | − | − | − | − | − | ++ | − | − | − | − |
| 2c | − | − | ++ | − | − | − | − | |||
| 3 | − | − | − | − | +++ | +++ | − | − | − | − |
| 4 | − | − | ++ | − | − | − | − | |||
| 5 | − | − | − | − | − | ++ | − | − | − | − |
| 6 | − | − | − | − | − | ++ | − | − | − | − |
| Strain | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| S. aureus B1 | 3 alone | OX alone | 3 with OX | OX with 3 | FIC index |
| ˃1024 | 128 | 64 | 16 | ˂0.188 * | |
| S. aureus B1 | 3 alone | AMP alone | 3 with AMP | AMP with 3 | FIC index |
| ˃1024 | 128 | ˃512 | 128 | ˃1.5 | |
3. Experimental Section
3.1. General Procedures
3.2. Extraction and Isolation
3.2.1. Similanpyrone A (1)
3.2.2. Similanpyrone B (2b)
3.2.3. Chevalone E (3)
3.2.4. Pyripyropene S (6)
3.3. X-ray Crystal Structure of Chevalone E (3)
3.4. Antimicrobial Activity Assays
3.4.1. Bacterial Strains
3.4.2. Determination of Minimum Inhibitory and Bactericidal/Fungal Concentrations
3.4.3. Synergistic Studies
3.4.3.1. Screening of Combined Effect between the Compounds and Antibiotics
3.4.3.2. Synergy Test: Checkerboard Method
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Asami, Y.; Kakeya, H.; Onose, R.; Yoshida, A.; Matsuzaki, H.; Osada, H. Azaspirene: A novel angiogenesis inhibitor containing a 1-oxa-7-azaspiro[4.4]non-2-ene-4,6-dione skeleton produced by the fungus Neosartorya sp. Org. Lett. 2002, 4, 2845–2848. [Google Scholar]
- Asami, Y.; Kakeya, H.; Onose, R.; Chang, Y.H.; Toi, M.; Osada, H. RK-805, an endothelial-cell-growth inhibitor produced by Neosartorya sp., and a docking model with methionine aminopeptidase-2. Tetrahedron 2004, 60, 7085–7091. [Google Scholar]
- Jayasuriya, H.; Zink, D.; Basilio, A.; Vicente, F.; Collado, J.; Bills, G.; Goldman, M.L.; Motyl, M.; Huber, J.; Dezeny, G.; et al. Discovery and antibacterial activity of glabramycin A–C from Neosartorya glabra by an antisense strategy. J. Antibiotics 2009, 62, 265–269. [Google Scholar]
- Yang, S.S.; Wang, G.J.; Cheng, K.F.; Chen, C.H.; Ju, Y.M.; Tsau, Y.J.; Lee, T.H. Bioactive γ-lactones from the fermented broth of Neosartorya sp. Planta Med. 2010, 76, 1701–1705. [Google Scholar] [CrossRef]
- Kijjoa, A.; Santos, S.; Dethoup, T.; Manoch, L.; Almeida, A.P.; Vasconcelos, M.H.; Silva, A.; Gales, L.; Herz, W. Sartoryglabrins, analogs of ardeemins, from Neosartorya glabra. Nat. Prod. Commun. 2011, 6, 807–812. [Google Scholar]
- Eamvijarn, A.; Kijjoa, A.; Bruyère, C.; Mathieu, V.; Manoch, V.; LeFranc, F.; Silva, A.; Kiss, R.; Herz, W. Secondary metabolites from a culture of the fungus Neosartorya pseudofischeri and their in vitro cytostatic activity in human cancer cells. Planta Med. 2012, 78, 1767–1776. [Google Scholar] [CrossRef]
- Buttachon, S.; Chandrapatya, A.; Manoch, L.; Silva, A.; Gales, L.; Bruyère, C.; Kiss, R.; Kijjoa, A. Sartorymensin, a new indole alkaloid, and new analogues of tryptoquivaline and fiscalins produced by Neosartorya siamensis (KUFC 6349). Tetrahedron 2012, 68, 3253–3262. [Google Scholar] [CrossRef]
- Eamvijarn, A.; Gomes, N. M.; Dethoup, T.; Buaruang, J.; Manoch, L.; Silva, A.; Pedro, M.; Marini, I.; Roussis, V.; Kijjoa, A. Bioactive meroditerpenes and indole alkaloids from the soil fungus Neosartorya fischeri (KUFC 6344), and the marine-derived fungi Neosartorya laciniosa (KUFC 7896) and Neosartorya tsunodae (KUFC 9213). Tetrahedron 2013, 69, 8583–8591. [Google Scholar] [CrossRef]
- Gomes, N.M.; Bessa, L.J.; Buttachon, S.; Costa, P.M.; Buaruang, J.; Dethoup, T.; Silva, A.M.S.; Kijjoa, A. Antibacterial and Antibiofilm activities of tryptoquivalines and meroditerpenes isolated from the marine-derived fungi Neosartorya paulistensis, N. laciniosa, N. tsunodae, and the Soil Fungi N. fischeri and N. siamensis. Mar. Drugs 2014, 12, 822–839. [Google Scholar]
- Kanokmedhakul, K.; Kanokmedhakul, S.; Suwannatrai, R.; Soytong, K.; Prabpai, S.; Kongsaeree, P. Bioactive meroterpenoids and alkaloids from the fungus Eurotium chevalieri. Tetrahedron 2011, 67, 5461–5468. [Google Scholar] [CrossRef]
- Ryoo, I.J.; Xu, G.H.; Km, Y.H.; Choo, S.J.; Ahn, J.S.; Bae, K.; Yoo, I.D. Reticulone, a novel free radical scavenger produced by Aspergillus sp. J. Microbiol. Biotechnol. 2009, 12, 1573–1575. [Google Scholar] [CrossRef]
- Gallo, M.B.C.; Cavalcanti, B.C.; Barros, F.W.A.; Moraes, M.O.; Costa-Latufo, L.V.; Pessoa, C.; Bastos, J.K.; Pupo, M.T. Chemical constituents of Papulaspora immersa, an endophyte from Smallanthus sonchifolius (Asteraceae), and their cytotoxic activity. Chem. Biodivers. 2010, 7, 2941–2950. [Google Scholar]
- Erkel, G.; Rether, J.; Anke, T. S14-95, a novel inhibitor of the JAK/STAT pathway from a Penicillium species. J. Antibiotics 2003, 56, 337–343. [Google Scholar] [CrossRef]
- Tomoda, H.; Tabata, N.; Yang, D.J.; Takaya.naki, H.; Nishida, M.; Omura, S. Pyripyropenes, novel ACAT inhibitors produced by Aspergillus fumigatus. III. Structure elucidation of pyripyropenes E to L. J. Antibiotics 1995, 48, 495–503. [Google Scholar]
- Obata, R.; Sunazuka, T.; Tomoda, H.; Harigaya, Y.; Omura, S. Chemical Modification and structure-activity relationships of pyripyropenes, potent, bioavailable inhibitor of acyl-CoA: cholesterol O-acyltransferase (ACAT). Bioorg. Med. Chem. Lett. 1995, 5, 2683–2688. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar]
- Franklin, R.; Cockerill, M.D., III. Performance Standards for Antimicrombial Susceptibility Testing. Twenty-First Informational Supplement M100-S21; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2011. [Google Scholar]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother 2003, 52, 1. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prompanya, C.; Dethoup, T.; Bessa, L.J.; Pinto, M.M.M.; Gales, L.; Costa, P.M.; Silva, A.M.S.; Kijjoa, A. New Isocoumarin Derivatives and Meroterpenoids from the Marine Sponge-Associated Fungus Aspergillus similanensis sp. nov. KUFA 0013. Mar. Drugs 2014, 12, 5160-5173. https://doi.org/10.3390/md12105160
Prompanya C, Dethoup T, Bessa LJ, Pinto MMM, Gales L, Costa PM, Silva AMS, Kijjoa A. New Isocoumarin Derivatives and Meroterpenoids from the Marine Sponge-Associated Fungus Aspergillus similanensis sp. nov. KUFA 0013. Marine Drugs. 2014; 12(10):5160-5173. https://doi.org/10.3390/md12105160
Chicago/Turabian StylePrompanya, Chadaporn, Tida Dethoup, Lucinda J. Bessa, Madalena M. M. Pinto, Luís Gales, Paulo M. Costa, Artur M. S. Silva, and Anake Kijjoa. 2014. "New Isocoumarin Derivatives and Meroterpenoids from the Marine Sponge-Associated Fungus Aspergillus similanensis sp. nov. KUFA 0013" Marine Drugs 12, no. 10: 5160-5173. https://doi.org/10.3390/md12105160