Aqueous Dried Extract of Skytanthus acutus Meyen as Corrosion Inhibitor of Carbon Steel in Neutral Chloride Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dried Extracts
2.2. Aqueous Extract General Characterization
2.3. Electrochemical Tests
- A 30 mL cell (SC) for linear sweep voltammetry (LSV) measurements under different DR dosing. The dosing was performed from a DR solution of 20 g/L of DR in 0.5 M NaCl. The LSV scan range was from −1000 to 0 mV/SHE at a scan rate of 2 mV/s. In this cell the WE were manufactured from a carbon steel rod with a diameter of 4.2 mm and a length of 10 mm. This was concentrically inserted using resin adhesive in a PTFE cylinder of 8 mm of diameter that serves a fixing device to the shaft of the rotating disk electrode cell stand (Figure 1a). Thus, the exposed area was a disk of 2.7 × 10−5 m2. The counter electrode was a platinum coil.
- A 200 mL cell (LC) adapted for a sequential measurement of LSV and electrochemical impedance spectroscopy (EIS). The WE were a carbon steel cylinder of 28 mm length and 13 mm diameter with a 9 mm threaded extension to be coupled to a motor shaft adaptor using an O-ring sealing, to keep the threaded section in dried condition while the exposed surface specimen is immersed in the electrolyte (Figure 1b). Before each test the WE were cleaned in an ultrasonic bath with distilled water then washed with isopropyl alcohol and acetone, dried at air, and finally weighed in an analytical scale. The exposed surface of this cylindrical WE were 1.27 × 10−3 m2. Using this WE design, LSV and EIS measurements were made at different immersion times along 48 h, combined with a global corrosion rate determination by the weight-loss method at the end of the run. For that purpose, the corroded WE were cleaned according to ASTM G1-90 to be weighed in an analytical scale.
2.4. Theory and Calculations
2.4.1. Electrochemical Parameters from LSV Data
Carbon Steel in Pure NaCl Solutions
Carbon Steel in DR Containing NaCl Solutions
2.4.2. Weight-Loss Experiments
3. Results and Discussion
3.1. Plant Extract Preparation
3.2. Early LSV Curves
3.3. Corrosion Inhibition under Unaerated Conditions Monitored by LSV and EIS at Different Immersion Times
3.4. Corrosion Inhibition under Aerated Conditions at Early Immersion Time
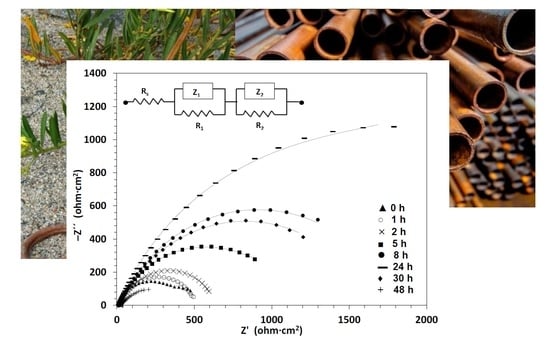
3.5. Corrosion Inhibition under Aerated Condition Monitored with LSV and EIS at Different Immersion Times
3.6. Corrosion Rate Density and Inhibition Efficiency Data for 24 h Runs at Three Different Rotation Rates and DR Dose
3.7. Corrosion Rate Density Evolution with Time: Global Comparison
3.7.1. Corrosion at Early Immersion Time
3.7.2. Corrosion Evolution with Different DR Dose
3.8. Dried Extract Characterization
3.9. Corrosion Inhibition Mechanism
- values for carbon steel in 0.5 M NaCl are similar to those obtained in the presence of the inhibitor. Also, gradually decrease with the immersion time;
- Partial polarization curves emerge from a mixed potential analysis that gradually evolve with time. The synthesized curves indicate that some components (shown in Figure 10) are electroactive at the potential range that carbon steel corrosion takes place;
- Forty one per cent of the dissolved iron from the corrosion process remained as film attached on the metal surface during 24 h corrosion runs with 600 ppm DR;
- At DR doses larger than 300 ppm, the dark color-evolving solutions remained translucent. Absence of precipitates was observed in solutions left to rest at room temperature for several months. Color formation together with corrosion inhibition takes place irrespective of the presence of if dissolved oxygen in solution. Thus, electroactive components in DR can either be oxidized or reduced at the expense of reduction or oxidation; and
- Combined EIS and LSV measurements demonstrated that film formation evolves with time, provides an increasing corrosion protection of carbon steel in chloride media, and after a limiting time when the film gradually degrades with a subsequent increase in values. This would suggest that after a phenolics- complex is formed a subsequent decomposition or detachment would take place.
4. Conclusions
- Dry aqueous extracts from Skytanthus acutus leaves, a native plant from the Atacama Desert in northern Chile, are active inhibitors of carbon steel corrosion in 0.5 M NaCl;
- The dry extract inhibitor conveys easy handling, stability, and storage for long period of time without degradation;
- From the mixed potential theory applied to polarization E-I data for carbon steel in NaCl solutions dosed with DR, the total current can be expressed as a superposition of partial current for hydrogen evolution, oxygen reduction, iron oxidation, and global redox reaction from component(s) present at the Skytanthus acutus extract;
- Based on the mixed potential theory it was demonstrated that a redox activity of the Skytanthus acutus dry extract components is involved in the corrosion inhibition mechanism of carbon steel in 0.5 M NaCl;
- The corrosion inhibition efficiency of doses larger than 300 ppm of the dry extract is not significantly affected by a rotation rate as high as 3000 rpm;
- The optimum DR dose for carbon steel inhibition is 600 ppm with a maximum inhibition efficiency of 90%;
- The corrosion inhibition efficiency gradually decreases with time when DR is added on a batch basis; and
- The experimental evidence indicated that the inhibition mechanisms can be formulated in terms of four stages of adsorption of organic ligands, iron dissolution, complex formation, and complex desorption.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| AISI 1020 | Steel low-tensile carbon steel |
| ASTM D2688–05 | Standard Test Methods for Corrosivity of Water in the Absence of Heat Transfer (Weight-Loss Methods) |
| ASTM G1-90 | Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens |
| HPLC-MS | Liquid Chromatography–Mass Spectrometry |
| UHPLC-PDA-HESI-orbitrap-MS/MS | Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry and a Photodiode Array Detector |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
| EIS | Electrochemical Impedance Spectroscopy |
| DR | Dried Residue |
| MS | Mass Spectrometry |
| AgCl | Silver Chloride |
| KCl | Potassium Chloride |
| NaCl | Sodium Chloride |
| HCl | Hydrochloric acid |
| Ag | Silver |
| C | Carbon |
| Mn | Manganese |
| Fe | Iron |
| S | Sulfur |
| Ni | Nickel |
| Cr | Chromium |
| Sn | Tin |
| P | Phosphorus |
| Mo | Molybdenum |
| N2 | Nitrogen gas |
| SC | Small Cell |
| LC | Large Cell |
| LSV | Linear Sweep Voltammetry |
| SHE | Standard Hydrogen Electrode |
| RPM | Revolutions per minute |
| PTFE | Polytetrafluoroethylene |
| WE | Working Electrode |
| HE | Hydrogen Evolution |
| ORR | Dissolved Oxygen Reduction |
| IO | Iron Oxidation |
| E | Potential |
| Total current density | |
| Partial Reduction Current for Dissolved Oxygen Reduction | |
| Partial reduction Current for Hydrogen Evolution | |
| Exchange Current Density for Dissolved Oxygen Reduction | |
| Exchange Current Density for Hydrogen Evolution | |
| Exchange Current Densities for Iron Oxidation | |
| Limiting Current Density | |
| Delta Limiting Current Density | |
| Total Current Density in Presence of Inhibitor | |
| Exchange Current Density in Presence of Inhibitor for Dissolved Oxygen Reduction | |
| Exchange Current Density in Presence of Inhibitor for Hydrogen Evolution | |
| Exchange Current Density in Presence of Inhibitor for Iron Oxidation | |
| Exchange Current Density in Presence of Inhibitor for Global Redox Reaction (plant-extract components) | |
| Inhibition Efficiency | |
| Corrosion Rates Density | |
| Corrosion Rates Density in Absence of the Inhibitor | |
| Corrosion Rates Density in Presence of the Inhibitor | |
| Overpotential for Dissolved Oxygen Reduction |
Appendix A
References
- Zhang, L.; He, Y.; Zhou, Y.; Yang, R.; Yang, Q.; Qing, D.; Niu, Q. A novel imidazoline derivative as corrosion inhibitor for P110 carbon steel in hydrochloric acid environment. Petroleum 2015, 1, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Cisternas, L.A.; Gálvez, E.D. The use of seawater in mining. Miner. Process. Extr. Metall. Rev. 2018, 39, 18–33. [Google Scholar] [CrossRef]
- Alvez, A.; Aitken, D.; Rivera, D.; Vergara, M.; McIntyre, N.; Concha, F. At the crossroads: Can desalination be a suitable public policy solution to address water scarcity in Chile’s mining zones? J. Environ. Manag. 2020, 258, 110039. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Chang, S.L.; Lee, S.L. Corrosion inhibition of steel by thiourea and cations under incomplete cathodic protection in a 3.5% NaCl solution and seawater. J. Appl. Electrochem. 1999, 29, 911–918. [Google Scholar] [CrossRef]
- Finšgar, M.; Petovar, B.; Xhanari, K.; Maver, U. The corrosion inhibition of certain azoles on steel in chloride media: Electrochemistry and surface analysis. Corros. Sci. 2016, 111, 370–381. [Google Scholar] [CrossRef]
- Othman, N.K.; Yahya, S.; Ismail, M.C. Corrosion inhibition of steel in 3.5% NaCl by rice straw extract. J. Ind. Eng. Chem. 2019, 70, 299–310. [Google Scholar] [CrossRef]
- Winkler, D.A.; Breedon, M.; Hughes, A.E.; Burden, F.R.; Barnard, A.S.; Harvey, T.G.; Cole, I. Towards chromate-free corrosion inhibitors: Structure-property models for organic alternatives. Green Chem. 2014, 16, 3349–3357. [Google Scholar] [CrossRef] [Green Version]
- Mahdavian, M.; Naderi, R. Corrosion inhibition of mild steel in sodium chloride solution by some zinc complexes. Corros. Sci. 2011, 53, 1194–1200. [Google Scholar] [CrossRef]
- Makarychev, Y.; Gladkikh, N.; Arkhipushkin, I.; Kuznetsov, Y. Corrosion inhibition of low-carbon steel by hydrophobic organosilicon dispersions. Metals 2021, 11, 1269. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green corrosion inhibitors from natural sources and biomass wastes. Molecules 2019, 24, 48. [Google Scholar] [CrossRef] [Green Version]
- Tolumoye, J.T.; Ezenwa, A.O.; Okechukwu, O.O. Corrosion inhibition of heat treated mild steel with Neem Leave extract in a chloride medium. Int. J. Res. Eng. Technol. 2015, 4, 404–409. [Google Scholar]
- Deyab, M.A. Inhibition activity of Seaweed extract for mild carbon steel corrosion in saline formation water. Desalination 2016, 384, 60–67. [Google Scholar] [CrossRef]
- Monticelli, C.; Frignani, A.; Trabanelli, G. Study on corrosion inhibitors for concrete application. Cem. Concr. Res. 2000, 30, 635–642. [Google Scholar] [CrossRef]
- Okeniyi, J.O.; Loto, C.A.; Popoola, A.P.I. Effects of Phyllanthus muellerianus leaf-extract on steel-reinforcement corrosion in 3.5% NaCl-immersed concrete. Metals 2016, 6, 255. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, R.; Marticorena, C.; Alarcón, D.; Baeza, C.; Cavieres, L.; Finot, V.L.; Fuentes, N.; Kiessling, A.; Mihoc, M.; Pauchard, A.; et al. Catálogo de las plantas vasculares de Chile. Gayana. Botánica 2018, 75, 1–430. [Google Scholar] [CrossRef] [Green Version]
- Cáceres, L.; Gómez-Silva, B.; Garró, X.; Rodríguez, V.; Monardes, V.; McKay, C.P. Relative humidity patterns and fog water precipitation in the Atacama Desert and biological implications. J. Geophys. Res. Biogeosci. 2007, 112, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Djerassi, C.; Kutney, J.P.; Shamma, M. Alkaloid Studies-XXXII. Studies on skytanthus acutus meyen. The structure of the monoterpenoid alkaloid skytanthine. Tetrahedron 1962, 18, 183–188. [Google Scholar] [CrossRef]
- Adolphen, G.; Appel, H.H.; Overton, K.H.; Warnock, W.D.C. Hydroxyskytanthines I and II. Two minor alkaloids of skytanthus acutus meyen. Tetrahedron 1967, 23, 3147–3150. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Areche, C.; Sepúlveda, B. Fast detection of phenolic compounds in extracts of easter pears (pyrus communis) from the atacama desert by ultrahigh-performance liquid chromatography and mass spectrometry (UHPLC-Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef] [Green Version]
- Simirgiotis, M.J.; Quispe, C.; Areche, C.; Sepúlveda, B. Phenolic compounds in chilean mistletoe (quintral, Tristerix tetrandus) analyzed by UHPLC-Q/Orbitrap/MS/MS and its antioxidant properties. Molecules 2016, 21, 241. [Google Scholar] [CrossRef] [Green Version]
- Flitt, H.J.; Schweinsberg, D.P. Synthesis, matching and deconstruction of polarization curves for the active corrosion of zinc in aerated near-neutral NaCl solutions. Corros. Sci. 2010, 52, 1905–1914. [Google Scholar] [CrossRef]
- Cáceres, L.; Vargas, T.; Parra, M. Study of the variational patterns for corrosion kinetics of carbon steel as a function of dissolved oxygen and NaCl concentration. Electrochim. Acta 2009, 54, 7435–7443. [Google Scholar] [CrossRef]
- Cáceres, L.; Herrera, L.; Vargas, T. Corrosion kinetics studies of AISI 1020 carbon steel from dissolved oxygen consumption measurements in aqueous sodium chloride solutions. Corrosion 2007, 63, 722–730. [Google Scholar] [CrossRef]
- Gimbun, J.; Pang, S.F.; Lau, S.J.; Yusoff, M.M.; Chuah, L. Thermal degradation kinetics of flavonoid derived from orthosiphon stamineus. Proc. Int. Conf. Food Prop. 2014, 1, 2–7. [Google Scholar]
- Turturică, M.; Stănciuc, N.; Bahrim, G.; Râpeanu, G. Investigations on sweet cherry phenolic degradation during thermal treatment based on fluorescence spectroscopy and inactivation kinetics. Food Bioprocess Technol. 2016, 9, 1706–1715. [Google Scholar] [CrossRef]
- Su, Y.L.; Leung, L.K.; Huang, Y.; Chen, Z.Y. Stability of tea theaflavins and catechins. Food Chem. 2003, 83, 189–195. [Google Scholar]
- Stănciuc, N.; Râpeanu, G. Kinetics of phytochemicals degradation during thermal processing of fruits beverages. Non-Alcohol. Beverages 2019, 6, 407–440. [Google Scholar]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Franzyk, H.; Frederiksen, S.M.; Jensen, S.R. Synthesis of monoterpene piperidines from the iridoid glucoside antirrhinoside. J. Nat. Prod. 1997, 60, 1012–1016. [Google Scholar] [CrossRef]
- Guin, P.S.; Das, S.; Mandal, P.C. Electrochemical reduction of quinones in different media: A review. Int. J. Electrochem. 2011, 2011, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Hilgemann, M.; Bassetto, V.; Kubota, L. Electrochemical approaches employed for sensing the antioxidant capacity exhibited by vegetal extracts: A review. Comb. Chem. High Throughput Screen. 2013, 16, 98–108. [Google Scholar] [PubMed]
- Kármán, F.H.; Felhösi, I.; Kálman, E.; Cserny, I.; Kövér, L. The role of oxide layer formation during corrosion inhibition of mild steel in neutral aqueous media. Electrochim. Acta 1998, 43, 69–75. [Google Scholar] [CrossRef]
- González, A.G.; Cadena-Aizaga, M.I.; Sarthou, G.; González-Dávila, M.; Santana-Casiano, J.M. Iron complexation by phenolic ligands in seawater. Chem. Geol. 2019, 511, 380–388. [Google Scholar] [CrossRef]
- Bommersbach, P.; Alemany-Dumont, C.; Millet, J.P.; Normand, B. Hydrodynamic effect on the behaviour of a corrosion inhibitor film: Characterization by electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 4011–4018. [Google Scholar] [CrossRef]
- Orlikowski, J.; Darowicki, K.; Mikołajski, S. Multi-sensor monitoring of the corrosion rate and the assessment of the efficiency of a corrosion inhibitor in utility water installations. Sens. Actuators B Chem. 2013, 181, 22–28. [Google Scholar] [CrossRef]
- Elhabiri, M.; Carrër, C.; Marmolle, F.; Traboulsi, H. Complexation of iron(III) by catecholate-type polyphenols. Inorg. Chim. Acta 2007, 360, 353–359. [Google Scholar] [CrossRef]
- Tuchagues, J.P.M.; Hendrickson, D.N. Iron(III) complexes with semiquinone and hydroquinone ligands. Inorg. Chem. 1983, 22, 2545–2552. [Google Scholar] [CrossRef]
- Torres, V.V.; Amado, R.S.; de Sá, C.F.; Fernandez, T.L.; de Sá Riehl, C.A.; Torres, A.G.; D’Elia, E. Inhibitory action of aqueous coffee ground extracts on the corrosion of carbon steel in HCl solution. Corros. Sci. 2011, 53, 2385–2392. [Google Scholar] [CrossRef]
- Nofrizal, S.; Rahim, A.A.; Saad, B.; Bothi Raja, P.; Shah, A.M.; Yahya, S. Elucidation of the corrosion inhibition of mild steel in 1.0 M HCl by catechin monomers from commercial green tea extracts. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 1382–1393. [Google Scholar] [CrossRef]
- Valek, L.; Martinez, S.; Mikulić, D.; Brnardić, I. The inhibition activity of ascorbic acid towards corrosion of steel in alkaline media containing chloride ions. Corros. Sci. 2008, 50, 2705–2709. [Google Scholar] [CrossRef]
- Ji, G.; Anjum, S.; Sundaram, S.; Prakash, R. Musa paradisica peel extract as green corrosion inhibitor for mild steel in HCl solution. Corros. Sci. 2015, 90, 107–117. [Google Scholar] [CrossRef]
- Ailiesei, G.L.; Ciobanu, M.; Balan, M.; Stavarache, C.; Barbes, L.; Nicolescu, A.; Deleanu, C. NMR detected metabolites in complex natural fluids. Quinic acid in apple juice. Ovidius Univ. Ann. Chem. 2015, 26, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Noubigh, A.; Abderrabba, M.; Provost, E. Temperature and salt addition effects on the solubility behaviour of some phenolic compounds in water. J. Chem. Thermodyn. 2007, 39, 297–303. [Google Scholar] [CrossRef]
- Tamura, H. The role of rusts in corrosion and corrosion protection of iron and steel. Corros. Sci. 2008, 50, 1872–1883. [Google Scholar] [CrossRef] [Green Version]
- Foley, R.T. Role of the chloride ion in iron corrosion. Corrosion 1970, 26, 58–70. [Google Scholar] [CrossRef]
- Veys-Renaux, D.; Reguer, S.; Bellot-Gurlet, L.; Mirambet, F.; Rocca, E. Conversion of steel by polyphenolic model molecules: Corrosion inhibition mechanism by rutin, esculin, esculetol. Corros. Sci. 2018, 136, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Theis, T.L.; Singer, P.C. Complexation of Iron(II) by organic matter and its effect on Iron(II) oxygenation. Environ. Sci. Technol. 1974, 8, 569–573. [Google Scholar] [CrossRef]
- Daugherty, E.E.; Gilbert, B.; Nico, P.S.; Borch, T. Complexation and redox buffering of Iron(II) by dissolved organic matter. Environ. Sci. Technol. 2017, 51, 11096–11104. [Google Scholar] [CrossRef]
- Orsetti, S.; Laskov, C.; Haderlein, S.B. Electron transfer between iron minerals and quinones: Estimating the reduction potential of the Fe(II)-goethite surface from AQDS speciation. Environ. Sci. Technol. 2013, 47, 14161–14168. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. Introduction to the iron oxides. In The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Cornell, R.M., Schwertmann, U., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2003; pp. 1–7. [Google Scholar]
- Xu, J.; Jordan, R.B. Kinetics and mechanism of the oxidation of 2,3-Dihydroxybenzoic acid by Iron(III). Inorg. Chem. 1988, 27, 4563–4566. [Google Scholar] [CrossRef]
- Hynes, M.J.; O’Coinceanainn, M. The kinetics and mechanisms of reactions of iron(III) with caffeic acid, chlorogenic acid, sinapic acid, ferulic acid and naringin. J. Inorg. Biochem. 2004, 98, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jordan, R.B. Kinetics and mechanism of the reaction of aqueous iron(III) with ascorbic acid. Inorg. Chem. 1990, 29, 2933–2936. [Google Scholar] [CrossRef]
- Chvátalová, K.; Slaninová, I.; Březinová, L.; Slanina, J. Influence of dietary phenolic acids on redox status of iron: Ferrous iron autoxidation and ferric iron reduction. Food Chem. 2008, 106, 650–660. [Google Scholar] [CrossRef]
- Uchimiya, M.; Stone, A.T. Redox reactions between iron and quinones: Thermodynamic constraints. Geochim. Cosmochim. Acta. 2006, 70, 1388–1401. [Google Scholar] [CrossRef]
- Anddjelkovic, M.; Vancamo, J.; Demeulenauer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Panias, D.; Taxiarchou, M.; Paspaliaris, I.; Kontopoulos, A. Mechanisms of dissolution of iron oxides in aqueous oxalic acid solutions. Hydrometallurgy 1996, 42, 257–265. [Google Scholar] [CrossRef]











| Solution Condition | Kinetic and Corrosion Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
A/m2 | A/m2 | A/m2 | A/m2 | mV/dec | mV/dec | mV/dec | mV/SHE | A/m2 | |
| Aerated | 0.1004 | −1.33 × 10−4 | 2524 | −8.9 | −206.2 | −184.7 | 71.8 | −237 | 1.24 |
| Unaerated | - | −1.18 × 10−4 | 12,308 | - | - | −181.1 | 119.5 | −585 | 0.16 |
| Method | Polarization Curves | Weight Loss | ||
|---|---|---|---|---|
| , h | 0 | 2 | 24 | 24 |
| , A/m2 | 0.46 | 0.04 | 0.09 | 0.19 |
| Immersion Time | EIS Parameters | |||
|---|---|---|---|---|
| , h | α | |||
| 0 | 18.5 | 743 | 6.5 × 10−4 | 0.64 |
| 2 | 35.2 | 1512 | 4.1 × 10−4 | 0.67 |
| 17 | 25.1 | 2252 | 6.0 × 10−4 | 0.62 |
| 24 | 20.1 | 4180 | 8.5 × 10−4 | 0.63 |
| DR Dosing, ppm | |
|---|---|
| 0 | −8.9 |
| 100 | −8.3 |
| 300 | −7.8 |
| 600 | −7.6 |
| 1600 | −7.4 |
| Method | Polarization Curves | Weight Loss | |||||
|---|---|---|---|---|---|---|---|
| With DR | Without DR | IE % | |||||
| , h | 0 | 2 | 24 | 48 | 48 | 48 | - |
| , A/m2 | 0.91 | 0.22 | 0.37 | 0.23 | 0.41 | 1.26 | 68 |
| Method | Polarization Curves | Weight Loss | ||||||
|---|---|---|---|---|---|---|---|---|
| With DR | Without DR | IE % | ||||||
| , h | 0 | 2 | 24 | 40 | 48 | 48 | 48 | - |
| , A/m2 | 1.17 | 0.16 | 0.06 | 0.11 | 0.97 | 0.17 | 1.26 | 87 |
| Immersion Time | EIS Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| , h | ||||||||
| 0 | 12.1 | 64.4 | 1.1 × 10−2 | 0.98 | 349.8 | 5.6 × 10−4 | 0.78 | 414.3 |
| 1 | 20.3 | 186.1 | 3.1 × 10−3 | 0.59 | 491.9 | 6.7 × 10−4 | 0.89 | 678.0 |
| 16 | 15.4 | 249.2 | 4.6 × 10−3 | 0.61 | 624.8 | 2.0 × 10−3 | 0.91 | 874.0 |
| 24 | 18.9 | 167.9 | 4.0 × 10−3 | 0.59 | 277.3 | 5.2 × 10−3 | 0.95 | 445.3 |
| 48 | 10.8 | 189.4 | 1.3 × 10−2 | 0.80 | 237.3 | 2.1 × 10−2 | 0.34 | 426.7 |
| Immersion Time | EIS Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| , h | ||||||||
| 0 | 9.5 | 90 | 8.1 × 10−4 | 0.99 | 388 | 1.2 × 10−3 | 0.72 | 478 |
| 1 | 14.2 | 37 | 7.6 × 10−4 | 0.75 | 453 | 3.9 × 10−4 | 0.84 | 490 |
| 2 | 24.0 | 166 | 5.9 × 10−4 | 0.8 | 445 | 6.6 × 10−4 | 0.82 | 611 |
| 5 | 10.0 | 200 | 1.7 × 10−3 | 0.74 | 951 | 6.7 × 10−4 | 0.71 | 1151 |
| 8 | 18.5 | 19 | 9.5 × 10−2 | 1 | 1794 | 4.2 × 10−4 | 0.72 | 1813 |
| 24 | 13.9 | 92 | 8.3 × 10−4 | 0.81 | 2953 | 3.8 × 10−4 | 0.78 | 3045 |
| 30 | 12.6 | 120 | 7.7 × 10−3 | 1 | 1466 | 4.5 × 10−4 | 0.71 | 1586 |
| 48 | 19.7 | 85 | 1.6 × 10−2 | 0.99 | 241 | 4.9 × 10−3 | 0.49 | 327 |
| Rotation Rate: 0 rpm | |||||
| DR dosing, ppm | 0 | 100 | 300 | 600 | 1600 |
| 0.419 | 0.170 | 0.154 | 0.047 | 0.056 | |
| IE, % | - | 59.3 | 63.2 | 88.7 | 86.7 |
| Rotation Rate: 1200 rpm | |||||
| DR dosing, ppm | 0 | 100 | 300 | 600 | 1600 |
| 1.260 | 0.972 | 0.572 | 0.248 | 0.294 | |
| IE, % | - | 22.8 | 54.6 | 80.3 | 76.7 |
| Rotation Rate: 3000 rpm | |||||
| DR dosing, ppm | 0 | 100 | 300 | 600 | 1600 |
| 2.091 | 2.007 | 0.550 | 0.410 | 0.229 | |
| IE, % | - | 4.0 | 73.7 | 80.4 | 89.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres, L.; Frez, Y.; Galleguillos, F.; Soliz, A.; Gómez-Silva, B.; Borquez, J. Aqueous Dried Extract of Skytanthus acutus Meyen as Corrosion Inhibitor of Carbon Steel in Neutral Chloride Solutions. Metals 2021, 11, 1992. https://doi.org/10.3390/met11121992
Cáceres L, Frez Y, Galleguillos F, Soliz A, Gómez-Silva B, Borquez J. Aqueous Dried Extract of Skytanthus acutus Meyen as Corrosion Inhibitor of Carbon Steel in Neutral Chloride Solutions. Metals. 2021; 11(12):1992. https://doi.org/10.3390/met11121992
Chicago/Turabian StyleCáceres, Luis, Yohana Frez, Felipe Galleguillos, Alvaro Soliz, Benito Gómez-Silva, and Jorge Borquez. 2021. "Aqueous Dried Extract of Skytanthus acutus Meyen as Corrosion Inhibitor of Carbon Steel in Neutral Chloride Solutions" Metals 11, no. 12: 1992. https://doi.org/10.3390/met11121992