Nanotoxicology of Metal Oxide Nanoparticles
Abstract
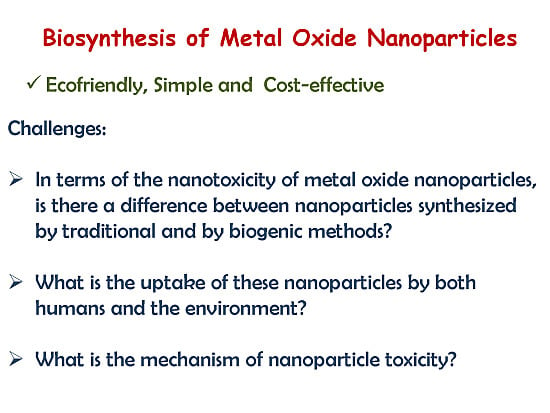
:1. Introduction

2. Biogenic Synthesis of Metal Oxide Nanoparticles
2.1. Bismuth Trioxide (Bi2O3) Nanocrystals
2.2. Cobalt Oxide (Co3O4) Nanocrystals
2.3. Copper Oxide (CuO, Cu2O) Nanoparticles
2.4. Iron Oxide (Fe2O3, Fe3O4) Magnetic Nanoparticles
2.5. Antimony Oxide (Sb2O3) Nanoparticles
2.6. Silica (SiO2) Nanoparticles
2.7. Titanium Dioxide (TiO2) Nanoparticles
2.8. Uraninite (UO2) Nanoparticles
2.9. Zinc Oxide (ZnO) Nanoparticles
2.10. Zirconia (ZrO2) Nanoparticles
2.11. Tin oxide (SnO2) Nanoparticles
3. Nanotoxicity of Metal Oxide Nanoparticles
3.1. Bismuth Trioxide (Bi2O3) Nanocrystals
3.2. Cobalt Oxide (Co3O4) Nanocrystals

3.3. Copper Oxide (CuO, Cu2O) Nanoparticles

3.4. Iron Oxide (Fe2O3, Fe3O4) Nanoparticles
3.5. Antimony Oxide (Sb2O3) Nanoparticles
3.6. Silica (SiO2) Nanoparticles

3.7. Titanium Dioxide (TiO2) Nanoparticles
3.8. Uraninite (UO2) Nanoparticles
3.9. Zinc Oxide (ZnO) Nanoparticles
3.10. Zirconia (ZrO2) Nanoparticles
3.11. Tin Oxide (SnO2) Nanoparticles
4. Relative Toxicity of Metal Oxide Nanoparticles

5. Final Remarks
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Bi2O3 | Hydrothermal process in assistance with the post-heat treatment route | Control of temperature impacts resulting products | Organic/toxic solvents and high temperatures | [17] |
| Bi2O3@PVA nanogels | Bi2O3 quantum dots in the interior of a nanogel of poly(vinyl alcohol) (PVA) | The nanogels can adapt to a surrounding fluids physiological temperature | Require inert atmosphere and irradiation with 60Co γ-ray source | [69] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Bi2O3 | Plant pathogenic fungus—Fusarium oxysporum | Room temperature, nanoparticles are stable in water | Necessity to investigate the fungus proteins on the surface of Bi2O3 | [19] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Co3O4 | Solvothermal route | Template-free approach | High temperature | [20] |
| Co3O4 | Thermal decomposition of molecular precursors derived from salicylic acid and cobalt (II) acetate or chloride | Template-free approach | High temperature | [21] |
| Co3O4 Nanoplates | Solid-state crystal re-construction route by conversion of hexagonal β-Co(OH)2 nanoplates | Template-free approach | Time consuming, high temperature | [22] |
| Co3O4 | Thermal decomposition | Control over size and shape | Toxicity to human cells and DNA damage | [70] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Co3O4 | Marine bacterium Brevibacterium casei | The protein coating on nanoparticles reduced agglomeration | Challenges to be faced: better control over size and crystallinity | [23 ] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| CuO, Cu2O | Thermal decomposition | Control over nanoparticle size and distribution | Costly in energy consumption | [136] |
| CuO | Electrospinning | Large scale production CuO | Time consumption | [137] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Cu2O | Baker’s yeast Saccharomyces cerevisiae | Room temperature no organic solvent | Challenges to be faced: better control over size and scaling up | [26] |
| CuO, Cu2O | Streptomyces sp. (Actinomycete biomass) | Environmentally friendly approach | Difficulties to obtain monodisperse nanoparticles and scaling up | [27] |
| CuO, Cu2O | Escherichia coli at aerobic condition | Neutral pH and room temperature | Necessity to investigate the bacterial proteins on the surface of nanoparticles | [28] |
| CuO, Cu2O | Penicillium aurantiogriseum, Penicillium citrinum and Penicillium waksmanii isolated from soil | Environmentally friendly approach | Low rate of synthesis, difficulties to obtain monodisperse nanoparticles. Microbial cultivation need to be improved | [29] |
| Cu2O | Tridax procumbens leaf extract | Simple, cost effective | Challenges to be faced: better control over size and scaling up | [30] |
| CuO | Aloe vera extract | Simple, cost effective | Challenges to be faced: better control over size and scaling up | [31] |
| CuO, Cu2O | White-rot fungus Stereum hirsutum | Simple method, under neutral or basic conditions | Scaling up and fungus cultivation | [138] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Fe3O4 | Co-precipitation | Relatively simple | Polydispersity Fe3O4 | [33] |
| Fe3O4 | Thermal decomposition of iron (III) acetylacetonate (Fe(acac)3) | Control of nanoparticle size and dispersibility | High temperature and inert atmosphere | [139] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Fe3O4 | Bacterium Actinobacter spp | Aerobic conditions | Limited scaling up, reaction time 24-48 h | [34] |
| Fe3O4 | Mycelia of acidophillic fungi, Verticillium sp. and Fusarium oxysporum | Extracellular synthesis | Limited scaling up, fungi cultivation | [35] |
| Fe2O3, Fe3O4 | Tannins from plants | Natural, nontoxic, and biodegradable polyphenolic compounds | Limited scaling up | [45] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Sb2O3 | γ-ray radiation-oxidation route | Control over size and distribution | Expensive, special equipment | [140] |
| Sb2O3 | Hydrothermal synthesis | Control over size and distribution | External pressure, high temperatures | [141] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Sb2O3 | Baker’s yeast (S. cerevisiae) | Low-cost, room temperature | Presence of nanoparticle aggregates | [48,49 ] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| SiO2 | Micelle-templated protocol by varying the silica source (tetra alkoxysilane with different alkoxy group) and the type and amounts of co-surfactant alcohols | Possibility to scaling up | Relatively wide particle size distribution, presence of contaminants | [142] |
| SiO2 | Surfactant template method source of silica tetra alkoxysilanes, and by varying the amounts of co-surfactant alcohols | Production of monodispersed spherical morphologies of nanoparticles | Time and energy consuming | [143] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| SiO2 | Fungus Fusarium oxysporum | Facile room temperature | Necessity to investigated the fungus secreted proteins involved in the synthesis | [53] |
| SiO2 | Bacterium Actinobacter sp | Particles were not cytotoxicity to human skin cells | Relatively time consuming reaction | [54] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| TiO2 | Hydrothermal growth using diethylamine as a passivating agent | Monodisperse nanoparticles with no phase transformation during the synthesis | Time and energy consuming | [144] |
| TiO2 | Sol-gel method under different pH conditions | Control over nanoparticle size | Toxic solvents, time and energy consuming | [145] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| TiO2 | Lactobacillus sp. (from yogurt and probiotic tablets) or Sachharomyces cerevisae (baker’s yeast) | Simple, room temperature and cost effective | Presence of few aggregates, difficult to scaling up | [56,57] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| UO2 | Radiolytic growth process in aqueous solutions through electron beam irradiation | Control over size distribution | Expensive, special equipment | [146] |
| UO2 | Hydrothermal synthesis method using hydrazine as a reducing agent | Free of surfactant or template or organic amines | Time and energy consuming | [147] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| UO2 | Dissimilatory metal- and sulfate-reducing bacteria Desulfovibrio desulfuricans | Simple, room temperature and cost effective | Microorganism growth | [58,59,60] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| ZnO | Combustion process, in which Zn(CH3COO)2 precursors migrated with the aid of alcoholic fuel to the top of a burning lampwick and the chemical reactions occurred at the solvent-air interface of the ignited lampwick | Relatively cost effective | ZnO exhibited a nonuniform size and shape | [148] |
| ZnO | Solvothermal synthesis | ZnO with good monodispersion in water | Organic toxic solvents | [149] |
| ZnO | Sol–gel processing technique based on hydrolysis of zinc acetate in methanol followed by supercritical drying in ethanol | Control over size and shape | Organic toxic solvents | [150] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| ZnO | Probiotic microbes Lactobacillus sporoge | Mild conditions and low-cost | Difficulties to scaling up | [64] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| ZrO2 | Sol–gel method | Nanoparticles with high chemical and structural homogeneity | Thermal treatment | [151] |
| ZrO2 | Thermal decomposition of the Zr(IV) complex as in presence of methanol and monoethylene glycol | Control over ZrO2 size and distribution | Organic/toxic solvents, high temperatures | [152] |
| ZrO2 | Thermal decomposition by zirconium oleate complex in a high boiling organic solvent | Production of oleophilic ZrO2 as nanofluilds | Organic/toxic solvents, high temperatures | [153] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| ZrO2 | Fungus Fusarium oxysporum | Extracellular hydrolysis, cost effect | Fungus cultivation and scaling up limitations | [66] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| SnO2 | Chemical precipitation using glycine which acts as a complexing agent and the surfactant sodium dodecyl sulfate as a stabilizing agent | Control over SnO2 size | Necessity to use surfactant and high temperature (up to 600 °C) | [154] |
| SnO2 | Solvothermal synthesis of SnO followed by its oxidation to SnO2 | Control over size and dispersion | Multiple steps, organic/toxic solvents | [155] |
| SnO2 | Reverse microemulsion method using different water to surfactant ratio | The size of the SnO2 can be tcontrolled by variation of water-to-surfactant ratio | Multiple steps, high temperature and necessity to sequential calcinations to remove the surfactant | [156] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| SnO2 | Saraca indica flower extract as a reducing agent | Simple, low cost | Scaling up | [67] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Bi2O3 | Hydrothermal process in assistance with the post-heat treatment route | Control of temperature impacts resulting products | Organic/toxic solvents and high temperatures | [17] |
| Bi2O3@PVA nanogels | Bi2O3 quantum dots in the interior of a nanogel of poly(vinyl alcohol) (PVA) | The nanogels can adapt to a surrounding fluids physiological temperature | Require inert atmosphere and irradiation with 60Co γ-ray source | [69] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Bi2O3 | Plant pathogenic fungus—Fusarium oxysporum | Room temperature, nanoparticles are stable in water | Necessity to investigate the fungus proteins on the surface of Bi2O3 | [19] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Co3O4 | Solvothermal route | Template-free approach | High temperature | [20] |
| Co3O4 | Thermal decomposition of molecular precursors derived from salicylic acid and cobalt (II) acetate or chloride | Template-free approach | High temperature | [21] |
| Co3O4 Nanoplates | Solid-state crystal re-construction route by conversion of hexagonal β-Co(OH)2 nanoplates | Template-free approach | Time consuming, high temperature | [22] |
| Co3O4 | Thermal decomposition | Control over size and shape | Toxicity to human cells and DNA damage | [70] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Co3O4 | Marine bacterium Brevibacterium casei | The protein coating on nanoparticles reduced agglomeration | Challenges to be faced: better control over size and crystallinity | [23] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| CuO, Cu2O | Thermal decomposition | Control over nanoparticle size and distribution | Costly in energy consumption | [136] |
| CuO | Electrospinning | Large scale production CuO | Time consumption | [137] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Cu2O | Baker’s yeast Saccharomyces cerevisiae | Room temperature no organic solvent | Challenges to be faced: better control over size and scaling up | [26] |
| CuO, Cu2O | Streptomyces sp. (Actinomycete biomass) | Environmentally friendly approach | Difficulties to obtain monodisperse nanoparticles and scaling up | [27] |
| CuO, Cu2O | Escherichia coli at aerobic condition | Neutral pH and room temperature | Necessity to investigate the bacterial proteins on the surface of nanoparticles | [28] |
| CuO, Cu2O | Penicillium aurantiogriseum, Penicillium citrinum and Penicillium waksmanii isolated from soil | Environmentally friendly approach | Low rate of synthesis, difficulties to obtain monodisperse nanoparticles. Microbial cultivation need to be improved | [29] |
| Cu2O | Tridax procumbens leaf extract | Simple, cost effective | Challenges to be faced: better control over size and scaling up | [30] |
| CuO | Aloe vera extract | Simple, cost effective | Challenges to be faced: better control over size and scaling up | [31] |
| CuO, Cu2O | White-rot fungus Stereum hirsutum | Simple method, under neutral or basic conditions | Scaling up and fungus cultivation | [138] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Fe3O4 | Co-precipitation | Relatively simple | Polydispersity Fe3O4 | [33] |
| Fe3O4 | Thermal decomposition of iron (III) acetylacetonate (Fe(acac)3) | Control of nanoparticle size and dispersibility | High temperature and inert atmosphere | [139] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Fe3O4 | Bacterium Actinobacter spp. | Aerobic conditions | Limited scaling up, reaction time 24-48 h | [34] |
| Fe3O4 | Mycelia of acidophillic fungi, Verticillium sp. and Fusarium oxysporum | Extracellular synthesis | Limited scaling up, fungi cultivation | [35] |
| Fe2O3, Fe3O4 | Tannins from plants | Natural, nontoxic, and biodegradable polyphenolic compounds | Limited scaling up | [45] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Sb2O3 | γ-ray radiation-oxidation route | Control over size and distribution | Expensive, special equipment | [140] |
| Sb2O3 | Hydrothermal synthesis | Control over size and distribution | External pressure, high temperatures | [141] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| Sb2O3 | Baker’s yeast (S. cerevisiae) | Low-cost, room temperature | Presence of nanoparticle aggregates | [48,49] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| SiO2 | Micelle-templated protocol by varying the silica source (tetra alkoxysilane with different alkoxy group) and the type and amounts of co-surfactant alcohols | Possibility to scaling up | Relatively wide particle size distribution, presence of contaminants | [142] |
| SiO2 | Surfactant template method source of silica tetra alkoxysilanes, and by varying the amounts of co-surfactant alcohols | Production of monodispersed spherical morphologies of nanoparticles | Time and energy consuming | [143] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| SiO2 | Fungus Fusarium oxysporum | Facile room temperature | Necessity to investigated the fungus secreted proteins involved in the synthesis | [53] |
| SiO2 | Bacterium Actinobacter sp. | Particles were not cytotoxicity to human skin cells | Relatively time consuming reaction | [54] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| TiO2 | Hydrothermal growth using diethylamine as a passivating agent | Monodisperse nanoparticles with no phase transformation during the synthesis | Time and energy consuming | [144] |
| TiO2 | Sol-gel method under different pH conditions | Control over nanoparticle size | Toxic solvents, time and energy consuming | [145] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| TiO2 | Lactobacillus sp. (from yogurt and probiotic tablets) or Sachharomyces cerevisae (baker’s yeast) | Simple, room temperature and cost effective | Presence of few aggregates, difficult to scaling up | [56,57] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| UO2 | Radiolytic growth process in aqueous solutions through electron beam irradiation | Control over size distribution | Expensive, special equipment | [146] |
| UO2 | Hydrothermal synthesis method using hydrazine as a reducing agent | Free of surfactant or template or organic amines | Time and energy consuming | [147] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| UO2 | Dissimilatory metal- and sulfate-reducing bacteria Desulfovibrio desulfuricans | Simple, room temperature and cost effective | Microorganism growth | [58,59,60] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| ZnO | Combustion process, in which Zn(CH3COO)2 precursors migrated with the aid of alcoholic fuel to the top of a burning lampwick and the chemical reactions occurred at the solvent-air interface of the ignited lampwick | Relatively cost effective | ZnO exhibited a nonuniform size and shape | [148] |
| ZnO | Solvothermal synthesis | ZnO with good monodispersion in water | Organic toxic solvents | [149] |
| ZnO | Sol–gel processing technique based on hydrolysis of zinc acetate in methanol followed by supercritical drying in ethanol | Control over size and shape | Organic toxic solvents | [150] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| ZnO | Probiotic microbes Lactobacillus sporoge | Mild conditions and low-cost | Difficulties to scaling up | [64] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| ZrO2 | Sol–gel method | Nanoparticles with high chemical and structural homogeneity | Thermal treatment | [151] |
| ZrO2 | Thermal decomposition of the Zr(IV) complex as in presence of methanol and monoethylene glycol | Control over ZrO2 size and distribution | Organic/toxic solvents, high temperatures | [152] |
| ZrO2 | Thermal decomposition by zirconium oleate complex in a high boiling organic solvent | Production of oleophilicZrO2 as nanofluilds | Organic/toxic solvents, high temperatures | [153] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| ZrO2 | Fungus Fusarium oxysporum | Extracellular hydrolysis, cost effect | Fungus cultivation and scaling up limitations | [66] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| SnO2 | Chemical precipitation using glycine which acts as a complexing agent and the surfactant sodium dodecyl sulfate as a stabilizing agent | Control over SnO2 size | Necessity to use surfactant and high temperature (up to 600 °C) | [154] |
| SnO2 | Solvothermal synthesis of SnO followed by its oxidation to SnO2 | Control over size and dispersion | Multiple steps, organic/toxic solvents | [155] |
| SnO2 | Reverse microemulsion method using different water to surfactant ratio | The size of the SnO2 can be controlled by variation of water-to-surfactant ratio | Multiple steps, high temperature and necessity to sequential calcinations to remove the surfactant | [156] |
| Nanoparticle | Route | Advantage | Disadvantage | Ref |
| SnO2 | Saraca indica flower extract as a reducing agent | Simple, low cost | Scaling up | [67] |
Acknowledgments
Conflicts of Interest
References
- Corr, S.A. Metal oxide nanoparticles. Nanoscience 2013, 1, 180–234. [Google Scholar]
- Haddad, P.S.; Seabra, A.B. Biomedical applications of magnetic nanoparticles. In Iron Oxides: Structure, Properties and Applications; Martinez, A.I., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; Volume 1, pp. 165–188. [Google Scholar]
- Durán, N.; Seabra, A.B. Metallic oxide nanoparticles: State of the art in biogenic syntheses and their mechanisms. Appl. Microbiol. Biotechnol. 2012, 95, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Haddad, P.S.; Duran, N. Biogenic synthesis of nanostructured iron compounds: Applications and perspectives. IET Nanobiotechnol. 2013, 7, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, O.; Rai, M.; Tortella, G.; Diez, M.C.; Seabra, A.B.; Durán, N. Biogenic nanoparticles: Copper, copper oxides, copper sulfides, complex copper nanostructures and their applications. Biotechnol. Lett. 2013, 35, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Kon, K.; Ingle, A.; Durán, N.; Galdiero, S.; Galdiero, M. Broad-spectrum Bioactivities of Silver Nanoparticles: The emerging trends and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Birla, S.; Gupta, I.; Ingle, A.; Gade, A.; Abd-Elsalam, K.; Marcato, P.D.; Durán, N. Diversity in synthesis and bioactivity of inorganic nanoparticles: Progress and pitfalls. Nanotechnol. Rev. 2014, 3, 281–309. [Google Scholar]
- Ingale, A.G.; Chaudhari, A.N. Biogenic synthesis of nanoparticles and potential applications: An eco-friendly approach. J. Nanomed. Nanotechol. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Guterres, S.S.; Alves, O.L. Nanotoxicology: Materials, Methodologies, and Assessments; Springer: New York, NY, USA, 2014; p. 412. [Google Scholar]
- Ingle, I.P.; Durán, N.; Rai, M. Bioactivity, mechanism of action and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- De Lima, R.; Seabra, A.B.; Durán, N. Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Feitosa, L.O.; Ballottin, D.; Marcato, P.D.; Tasic, L.; Durán, N. Cytotoxicity and genotoxicity of biogenic silver nanoparticles. J. Phys. Conf. Ser. 2013, 429, 012020. [Google Scholar] [CrossRef]
- Schrofel, A.; Kratosova, G.; Safarik, I.; Safarikova, M.; Raska, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles—A review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.-T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yang, C.; Wang, Z.; Zhou, W.; Jiao, S.; Zhu, H. In situ synthesis of α-β phase heterojuntion on Bi2O3 nanowires with execptional visible-light photocatalytic performance. Appl. Catal. B Environ. 2013, 142–143, 504–511. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Huang, L.; Xu, H.; Zhang, R.; Qu, M.; Gao, Q.; Li, H. g-C3N4 modified Bi2O3 composites with enhanced visible-light photocatalytic activity. J. Phys. Chem. Solids 2015, 76, 112–119. [Google Scholar] [CrossRef]
- Uddin, I.; Adhynthaya, S.; Syed, A.; Selvaraj, K.; Ahmad, A.; Poddar, P. Structure and microbial synthesis of sub-10 nm Bi2O3 nanocrystals. J. Nanosci. Nanotechnol. 2008, 8, 3909–3913. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yuan, F.; Yao, M.; Bang, J.H.; Lee, J.-H. A new synthetic route to hollow Co3O4 octahedra for supercapacitor applications. Cryst. Eng. Comm. 2014, 16, 826–833. [Google Scholar] [CrossRef]
- Hosny, N.M. Single crystalline Co3O4: Synthesis and optical properties. Mater. Chem. Phys. 2014, 144, 247–251. [Google Scholar] [CrossRef]
- Su, D.; Xie, X.; Munroe, P.; Dou, S.; Wang, G. Mesoporous hexagonal Co3O4 for high performance lithium ion batteries. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Shete, A.; Harle, A.S.; Kasyutich, O.; Schwarzacher, W.; Pundle, A.; Poddar, P. Extracellular bacterial synthesis of proteinfunctionalized ferromagnetic Co3O4 nanocrystals and imaging of self-organization of bacterial cells under stress after exposure to metal ions. Chem. Mater. 2008, 20, 1484–1491. [Google Scholar] [CrossRef]
- Kanhed, P.; Birla, S.; Gaikwad, S.; Gade, A.; Seabra, A.B.; Rubilar, O.; Duran, N.; Rai, M. In vitro antifungal efficacy of copper nanoparticles against selected crop phatogenic fungi. Mater. Lett. 2014, 115, 13–17. [Google Scholar] [CrossRef]
- Hasan, S.S.; Singh, S.; Parikh, R.Y.; Dharne, M.S.; Patole, M.S.; Prasad, B.L.V.; Shouche, Y.S. Bacterial synthesis of copper/copper oxide nanoparticles. J. Nanosci. Nanotechnol. 2008, 8, 3191–3196. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Jha, A.K.; Prasad, K.; Kulkarni, A.R. Can microbes mediate nano-transformation? Indian J. Phys. 2010, 84, 1355–1360. [Google Scholar] [CrossRef]
- Usha, R.; Prabu, E.; Palaniswamy, M.; Venil, C.K.; Rajendran, K.R. Synthesis of metal oxide nano particles by Streptomyces sp. for development of antimicrobial textiles. Global J. Biotechnol. Biochem. 2010, 5, 153–160. [Google Scholar]
- Singh, A.V.; Patil, R.; Anand, A.; Milani, P.; Gade, W.N. Biological synthesis of copper oxide nano particles using Escherichia coli. Curr. Nanosci. 2010, 6, 365–369. [Google Scholar] [CrossRef]
- Honary, S.; Barabadi, H.; Gharaeifathabad, E.; Naghibi, F. Green synthesis of copper oxide nanoparticles using Penicillium aurantiogriseum, Penicillium citrinum and Penicillium waksmanii. Digest J. Nanomat. Biostruct. 2012, 7, 999–1005. [Google Scholar]
- Gopalakrishnan, K.; Ramesh, C.; Ragunathan, V.; Thamilselvan, M. Antibacterial activity of Cu2O nanoparticles on E. coli synthesized from Tridax procumbens leaf extract and surface coating with polyaniline. Digest J. Nanomat. Biostruct. 2012, 7, 833–839. [Google Scholar]
- Sangeetha, G.; Rajeshwari, S.; Rajendran, V. Aloe barbadensis Miller mediated green synthesis of mono-disperse copper oxide nanoparticles: Optical properties. Spectrochim. Acta Part A 2012, 97, 1140–1144. [Google Scholar]
- Seabra, A.B.; Pasquoto, T.; Ferrarini, A.C.F.; Cruz, M.; Haddad, P.S.; de Lima, R. Preparation, characterization, cytotoxicity and genotoxicity evaluations of thiolated- and S-nitrosated superparamagnetic iron oxide nanoparticles: Implications for cancer treatment. Chem. Res. Toxicol. 2014, 27, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.M.; Seabra, A.B.; de Oliveira, M.G.; Itri, R.; Haddad, P.S. Nitric oxide donor superparamagnetic iron oxide nanoparticles. Mat. Sci. Eng. C 2013, 33, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Bharde, A.; Wani, A.; Shouche, Y.; Prasad, B.L.V.; Sastry, M. Bacterial aerobic synthesis of nanocrystalline magnetite. J. Am. Chem. Soc. 2005, 127, 9326–9327. [Google Scholar] [CrossRef] [PubMed]
- Bharde, A.; Rautaray, D.; Bansal, V.; Ahmad, A.; Sarkar, I.; Yusuf, S.M.; Sanyal, M.; Sastry, M. Extracellular biosynthesis of magnetite using fungi. Small 2006, 2, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Roh, Y.; Kim, K.W.; Hur, H.-G. Organic acid-dependent iron mineral formation by a newly isolated iron reducing bacterium, Shewanella sp. HN-41. Geomicrobiol. J. 2007, 24, 31–34. [Google Scholar] [CrossRef]
- Ceci, P.; Chiancone, E.; Kasyutich, O.; Bellapadrona, G.; Castelli, L.; Fittipaldi, M.; Gatteschi, D.; Innocenti, C.; Sangregorio, C. Synthesis of iron oxide nanoparticles in Listeria innocua Dps (DNA-binding protein from starved cells): A study with the wild-type protein and a catalytic centre mutante. Chem. Eur. J. 2010, 16, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Yaaghoobi, M.; Emtiazi, G.; Roghanian, R. A novel approach for aerobic construction of iron oxide nanoparticles by Acinetobacter radioresistens and their effects on red blood cells. Curr. Nanosci. 2012, 8, 286–291. [Google Scholar] [CrossRef]
- Raikher, Y.L.; Stepanov, V.I.; Stolyar, S.V.; Ladygina, V.P.; Balaev, D.A.; Ishchenko, L.A.; Balasoiu, M. Magnetic properties of biomineral nanoparticles produced by bacteria Klebsiella oxytoca. Phys. Solid State 2010, 52, 298–305. [Google Scholar] [CrossRef]
- Stolyar, S.V.; Bayukov, O.A.; Gurevich, Y.L.; Denisova, E.A.; Iskhakov, R.S.; Ladygina, V.P.; Puzyr, A.P.; Pustoshilov, P.P.; Bitekhtina, M.A. Iron-containing nanoparticles from microbial metabolismo. Inorg. Mater. 2006, 42, 763–768. [Google Scholar] [CrossRef]
- Balasoiu, M.; Stolyar, S.V.; Iskhakov, R.S.; Ishchenko, L.A.; Raikher, Y.L.; Kuklin, A.I.; Orelovich, O.L.; Kovalev, Y.S.; Kurkin, T.S.; Arzumanian, G.M. Hierarchical structure investigations of biogenic ferrihydrite samples. Rom. J. Phys. 2010, 55, 782–789. [Google Scholar]
- Bazylinski, D.A.; Frankel, R.B.; Konhauser, K.O. Modes of biomineralization of magnetite by microbes. Geomicrobiol. J. 2007, 24, 456–475. [Google Scholar] [CrossRef]
- Byrne, J.M.; Telling, N.D.; Coker, V.S.; Pattrick, R.A.D.; van der Lann, G.; Arenholz, E.; Tuna, F.; Lloyd, J.R. Control of nanoparticle size, reactivity and magnetic properties during the bioproduction of magnetite by Geobacter sulfurreducens. Nanotechnology 2011, 22, 455709. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kim, J.W.; Choi, H.; Lee, J.-H.; Hur, H.-G. Synthesis of nanosized biogenic magnetite and comparison of its catalytic activity in ozonation. Appl. Catal. B 2008, 83, 208–213. [Google Scholar] [CrossRef]
- Herrera-Becerra, R.; Rius, J.L.; Zorrilla, C. Tannin biosynthesis of iron oxide nanoparticles. Appl. Phys. A 2010, 100, 453–459. [Google Scholar] [CrossRef]
- Andjelkovic, M.; van Camp, J.; de Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron–chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Vukovic, M.; Brankovic, Z.; Poleti, D.; Recnik, A.; Brankovic, G. Novel simple methods for the synthesis of single-phase valentinite Sb2O3. J. Sol-Gel Sci. Technol. 2014, 72, 527–533. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Prasad, K. Biosynthesis of Sb2O3 nanoparticles: A low-cost green approach. Biotechnol. J. 2009, 4, 1582–1585. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Prasad, K.; Prasad, K. A green low-cost biosynthesis of Sb2O3 nanoparticles. Biochem. Eng. J. 2009, 43, 303–306. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Liao, L.M. Applications of nanoparticles for anticancer drug delivery: A review. J. Nanosci. Nanotechnol. 2015, 7, 4753–4773. [Google Scholar] [CrossRef]
- Seabra, A.B.; Duran, N. Nitric oxide-releasing vehicles for biomedical applications. J. Mat. Chem. 2010, 20, 1664–1637. [Google Scholar] [CrossRef]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Bansal, V.; Rautaray, D.; Bharde, A.; Ahire, K.; Sanyal, A.; Ahmad, A.; Sastry, M. Fungus-mediated biosynthesis of silica and titania particles. J. Mater. Chem. 2005, 15, 2583–2589. [Google Scholar] [CrossRef]
- Singh, S.; Bhatta, U.M.; Satyam, P.V.; Dhawan, A.; Sastry, M.; Prasad, B.L.V. Bacterial synthesis of silicon/silica nanocomposites. J. Mater. Chem. 2008, 18, 2601–2606. [Google Scholar] [CrossRef]
- Goh, P.S.; Ng, B.C.; Lau, W.J.; Ismail, A.F. Inorganic nanomaterials in polymeric ultrafiltration membranes for water treatment. Sep. Purif. Rev. 2015, 44, 216–249. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K. Biosynthesis of metal and oxide nanoparticles using Lactobacilli from yoghurt and probiotic spore tablets. Biotechnol. J. 2010, 5, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Prasad, K.; Kulkarni, A.R. Synthesis of TiO2 nanoparticles using microorganisms. Colloids Surf. B Biointerf. 2009, 71, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Phillips, E.J.P. Bioremediation of uranium contamination with enzymatic uranium reduction. Environ. Sci. Technol. 1992, 26, 2228–2234. [Google Scholar] [CrossRef]
- Lovley, D.; Phillips, E.J.P. Reduction of uranium by Desulfovibrio desulfuricans. Appl. Environ. Microbiol. 1992, 58, 850–856. [Google Scholar] [PubMed]
- Tebo, B.M.; Obraztsova, A.Y. Sulfate-reducing bacterium grows with Cr(VI), U(VI), Mn(IV), and Fe(III) as electron acceptors. FEMS Microbiol. Lett. 1998, 16, 193–198. [Google Scholar] [CrossRef]
- Ulrich, K.U.; Ilton, E.S.; Veeramani, H.; Sharp, J.O.; Bernier-Latmani, R.; Schofield, E.J.; Bargar, J.R.; Giammar, D.E. Comparative dissolution kinetics of biogenic and chemogenic uraninite under oxidizing conditions in the presence of carbonate. Geochim. Cosmochim. Acta 2009, 73, 6065–6083. [Google Scholar] [CrossRef]
- Burgos, W.D.; McDonough, J.T.; Senko, J.M.; Zhang, G.X.; Dohnalkova, A.C.; Kelly, S.D.; Gorby, Y.; Kemner, K.M. Characterization of uraninite nanoparticles produced by Shewanella oneidensis MR-1. Geochim. Cosmochim. Acta 2008, 72, 4901–4915. [Google Scholar] [CrossRef]
- Singer, D.M.; Farges, F.; Brown, G.E., Jr. Biogenic nanoparticulate UO2: Synthesis, characterization, and factors affecting surfase reactivity. Geochim. Cosmochim. Acta 2009, 73, 3593–3611. [Google Scholar] [CrossRef]
- Prasad, K.; Jha, A.K. ZnO nanoparticles: synthesis and adsorption study. Natural Sci. 2009, 1, 129–135. [Google Scholar] [CrossRef]
- Somiya, S.; Yamamoto, N.; Yanagina, H. Science and Tecnology of Zirconia III (Advances in Ceramics); American Ceraminc Society: Westerville, OH, USA, 1988; Volumes 24A and 24B. [Google Scholar]
- Bansal, V.; Rautaray, D.; Ahmad, A.; Sastry, M. Biosynthesis of zirconia nanoparticles using the fungus Fusarium oxysporum. J. Mater. Chem. 2004, 14, 3303–3305. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Philip, D. Biogenic synthesis of SnO2 nanoparticles: Evaluation of antibacterial and antioxidant activities. Spectrochim. Acta Part A Mol. Biomol. Spec. 2015, 134, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Bismuth Trioxide Toxicology. Available online: http://digitalfire.com/4sight/hazards/ceramic_hazard_bismuth_trioxide_toxicology_352.html (accessed on 28 May 2015).
- Zhu, H.; Li, Y.; Qiu, R.; Shi, L.; Wu, W.; Zhou, S. Responsive fluorescent Bi2O3@PVA hybrid nanogels for temperature-sensing, dual-modal imaging, and drug delivery. Biomaterials 2012, 33, 3058–3069. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Dash, S.K.; Tripathy, S.; Das, B.; Mandal, D.; Pramanik, P.; Roy, S. Toxicity of cobalt oxide nanoparticles to normal cells; an in vitro and in vivo study. Chem.-Biol. Inter. 2015, 226, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Papis, E.; Gornati, R.; Prati, M.; Ponti, J.; Sabbioni, E.; Bernardini, G. Gene expression in nanotoxicology research: Analysis by differential display in BALB3T3 fibroblasts exposed to cobalt particles and ions. Toxicol. Lett. 2007, 107, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Ponti, J.; Sabbioni, E.; Munaro, B.; Broggi, F.; Marmorato, P.; Franchini, F.; Colognato, R.; Rossi, F. Genotoxicity and morphological transformation induced by cobalt nanoparticles and cobalt chloride: An in vitro study in Balb/3T3 mouse fibroblasts. Mutagenesis 2009, 24, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Colognato, R.; Bonelli, A.; Ponti, J.; Farina, M.; Bergamaschi, E.; Sabbioni, E.; Migliore, L. Comparative genotoxicity of cobalt nanoparticles and ions on human peripheral leukocytes in vitro. Mutagenesis 2008, 23, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.; Bresson, C.; Darolles, C.; Gautiers, C.; Roudeau, S.; Perrin, L.; Janin, M.; Floriani, M.; Aloin, C.; Carmona, A.; et al. Low-solubility particles and a Trojan-horse type mechanism of toxicity: The case of cobalt oxide on human lung cells. Part. Fibre Toxicol. 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Papis, E.; Rossi, F.; Raspanti, M.; Dalle-Donne, I.; Colombo, G.; Milzani, A.; Bernardini, G.; Gornati, R. Engineered cobalt oxide nanoparticles readily enter cells. Toxicol. Lett. 2009, 189, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-S.; Dart, K.; Nowakowska, D.J.; Zheng, X.; Donaldson, K.; Howie, S.E.M. Adjuvanticity and toxicity of cobalt oxide nanoparticles as an alternative vaccine adjuvant. Nanomedicine 2012, 7, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Culloty, S.; Darmody, G.; Lynch, S.; Davenport, J.; Ramirez-Garcia, S.; Dawson, K.A.; Lynch, I.; Blasco, J.; Sheehan, D. Toxicity of copper oxide nanoparticles in the blue mussel, Mytilus edulis: A redox proteomic investigation. Chemosphere 2014, 108, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Heinlaan, M.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Pramanik, A.; Maity, J.; Mukherjee, A.; Pramanik, P.; Laskar, A.; Karmakar, P. Interplay between autophagy and apoptosis mediated by copper oxide nanoparticles in human breast cancer cells MCF7. Biochim Biophys Acta 2014, 1840, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Alhadlaq, H.A.; Ahmad, J.; Al-Khedhairy, A.A.; Musarrat, J.; Ahamed, M. Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS ONE 2013, 8, e69534. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yan, Y.; Zhao, Y.; Guo, F.; Jiang, C. Copper oxide nanoparticles induce autophagic cell death in A549 cells. PLoS ONE 2012, 7, e43442. [Google Scholar] [CrossRef] [PubMed]
- Padil, V.V.T.; Cerník, M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Inter. J. Nanomed. 2013, 8, 889–898. [Google Scholar]
- Abboud, Y.; Saffaj, T.; Chagraoui, A.; Bouari, E.; Brouzi, K.; Tanane, O.; Ihssane, B. Biosynthesis, characterization and antimicrobial activity of copperoxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata). Appl. Nanosci. 2014, 4, 571–576. [Google Scholar]
- Acharyulu, N.P.S.; Dubey, R.S.; Swaminadham, V.; Kollu, P.; Kalyani, R.L.; Pammi, S.V.N. Green Synthesis of CuO Nanoparticles using Phyllanthus amarus Leaf Extract and their Antibacterial Activity against Multidrug Resistance Bacteria. Inter. J. Eng. Res. Technol. 2014, 3, 639–641. [Google Scholar]
- Sivaraj, R.; Rahman, P.K.S.M.; Rajiv, P.; Venckatesh, H.A.S.R. Biogenic copper oxide nanoparticles synthesis using Tabernaemontana divaricate leaf extract and its antibacterial activity against urinary tract pathogen. Spectrochim. Acta Part A Mol. Biomol. Spec. 2014, 133, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Haddad, P.S. Cytotoxicity and Genotoxicity of Iron Oxides Nanoparticles. In Nanotoxicology: Materials, Methodologies, and Assessment; Durán, N., Guterres, S.S., Alves, O.L., Eds.; Springer: New York, NY, USA, 2014; Chapter 12; pp. 265–279. [Google Scholar]
- Xiang, L.; Wei, J.; Jianbo, S.; Guili, W.; Feng, G.; Ying, L. Purified and sterilized magnetosomes from Magnetospirillum gryphiswaldense MSR-1 were not toxic to mouse fibroblasts in vitro. Lett. Appl. Microbiol. 2007, 45, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Schuler, D. Biogenic nanoparticles: Production, characterization, and application of bacterial magnetosomes. J. Phys. Condens. Matter. 2006, 18, S2815–S2828. [Google Scholar] [CrossRef]
- De Lima, R.; Oliveira, J.L.; Ludescher, A.; Molina, M.M.; Itri, R.; Seabra, A.B.; Haddad, P.S. Nitric oxide releasing iron oxide magnetic nanoparticles for biomedical applications: Cell viability, apoptosis and cell death evaluations. J. Phys. Conf. Ser. 2013, 429, 012034. [Google Scholar] [CrossRef]
- De Lima, R.; Oliveira, J.L.; Murakami, P.S.K.; Molina, M.M.; Itri, R.; Haddad, P.S.; Seabra, A.B. Iron oxide nanoparticles show no toxicity in the comet assay in lymphocytes: A promising vehicle as a nitric oxide releasing nanocarriers in biomedical applications. J. Phys. Conf. Ser. 2013, 429, 012021. [Google Scholar] [CrossRef]
- Wu, H.; Yin, J.-J.; Wamer, W.G.; Zeng, M.; Lo, Y.M. Reactive oxygen species-related activities of nano-iron metal and nano-iron oxides. J. Food Drug Anal. 2014, 22, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, S.; Yang, Y.; Zhao, F.; Huang, J.; Chang, J. Comparison of magnetite nanocrystals formed by biomineralization and chemosynthesis. J. Mag. Mag. Mat. 2007, 313, 236–242. [Google Scholar] [CrossRef]
- Ross, G.; Harrison, A.P. The exposure to and health effects of antimony. Indian J. Occup. Environ. Med. 2009, 13, 3–10. [Google Scholar]
- Bregoli, L.; Francesca, C.; Gambarelli, A.; Sighinolfi, G.; Gatti, A.M.; Santi, P.; Martelli, A.M.; Cocco, L. Toxicity of antimony trioxide nanoparticles on human hematopoietic progenitor cells and comparison to cell lines. Toxicology 2009, 262, 121–129. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.; Inkielewicz-Stepniak, I.; Corbalan, J.J.; Radomski, M.M. Mechanisms of toxicity of amorphous silica nanoparticles on human Lung submucosal cells in vitro: Protective effects of Fisetin. Chem. Res. Toxicol. 2012, 25, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Bancos, S.; Stevens, D.L.; Tyner, K.M. Effect of silica and gold nanoparticles on macrophage proliferation, activation markers, cytokine production, and phagocytosis in vitro. Int. J. Nanomed. 2015, 10, 183–206. [Google Scholar]
- Hassankhani, R.; Esmaeillou, M.; Tehrani, A.A.; Nasirzadeh, K.; Khadir, F.; Maadi, H. In vivo toxicity of orally administrated silicon dioxide nanoparticles in healthy adult mice. Environ. Sci. Pollut. Res. 2015, 22, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-R.; Lee, S.-Y.; Lee, E.J.; Park, S.H.; Seong, N.; Seo, H.-S.; Shin, S.S.; Kim, S.J.; Meang, E.H.; Park, M.K.; et al. Toxicity of colloidal silica nanoparticles administered orally for 90 days in rats. Int. J. Nanomed. 2014, 9, 67–78. [Google Scholar]
- Periasamy, V.S.; Athinarayanan, J.; Akbarsha, M.A.; Alshatwi, A.A. Silica nanoparticles induced metabolic stress through EGR1, CCND, and E2F1 genes in human mesenchymal stem cells. Appl. Biochem. Biotechnol. 2015, 175, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, Y. Lung injury induced by TiO2 nanoparticles depends on their structural features: size, shape, crystal phases, and surface coating. Int. J. Mol. Sci. 2014, 15, 22258–22278. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Wang, L.; Sang, X.; Zhao, X.; Hong, J.; Cheng, S.; Yu, X.; Liu, D.; Xu, B.; Hu, R.; et al. Nano-sized titanium dioxide-induced splenic toxicity: A biological pathway explored using microarray technology. J. Hazard. Mat. 2014, 278, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Órdenes-Aenishanslins, N.A.; Saona, L.A.; Durán-Toro, V.M.; Monrás, J.P.; Bravo, D.M.; Pérez-Donoso, J.M. Use of titanium dioxide nanoparticles biosynthesized by Bacillus mycoides in quantum dot sensitized solar cells. Microb. Cell Fact. 2014, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, G.; Rahuman, A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.; Velayutham, K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta Part A 2012, 91, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Jayaseelan, C.; Rahuman, A.; Roopan, S.M.; Kirthi, A.V.; Venkatesan, J.; Kim, S.K.; Iyappan, M.; Siva, C. Biological approach to synthesize TiO2 nanoparticles using Aeromonas hydrophila and its antibacterial activity. Spectrochim Acta Part A 2013, 107, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Babitha, S.; Korrapati, P.S. Biosynthesis of titanium dioxide nanoparticles using a probiotic from coal fly ash effluent. Mater. Res. Bull. 2013, 48, 4738–4742. [Google Scholar] [CrossRef]
- Documentation of the Threshold Limit Values and Biological Exposure Indices, 6th ed.; American Conference of Governmental Industrial Hygienists Inc: Cincinnati, OH, USA, 1991.
- Craig, D.K. Chemical and radiological toxicity of uranium and its compounds. WSRC-TR-2001–00331. Available online: http://sti.srs.gov/fulltext/tr2001331/tr2001331.html (accessed on 28 May 2015).
- Petitot, F.; Lestaevel, P.; Tourlonias, E.; Mazzucco, C.; Jacquinot, S.; Dhieux, B.; Delissen, O.; Tournier, B.B.; Gensdarmes, T.F.; Beaunier, P.; et al. Inhalation of uranium nanoparticles: Respiratory tract deposition and translocation to secondary target organs in rats. Toxicol. Lett. 2013, 217, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Monleau, M.; de Meo, M.; Frelon, S.; Paquet, F.; Donnadieu-Claraz, M.; Duménil, G.; Chazel, V. Distribution and genotoxic effects after successive exposure to different uranium oxide particles inhaled by rats. Inhal. Toxicol. 2006, 18, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Baik, M.H.; Choi, J.W. Biogenic formation and growth of uraninite (UO2). Environ. Sci. Technol. 2010, 44, 8409–8414. [Google Scholar] [CrossRef] [PubMed]
- Newsome, L.; Morris, K.; Jonathan, R.; Lloyd, J.R. The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chem. Geol. 2014, 363, 164–184. [Google Scholar] [CrossRef]
- Baskar, G.; Chandhuru, J.; Fahad, K.S.; Praveen, A.S. Mycological synthesis, characterization and antifungal activity of zinc oxide nanoparticles. Asian J. Pharm. Tech. 2013, 3, 142–146. [Google Scholar]
- AbdElhady, M.M. Preparation and characterization of chitosan/zinc oxide nanoparticles for imparting antimicrobial and UV protection to cotton fabric. Int. J. Carbohy. Chem. 2012, 840591. [Google Scholar] [CrossRef]
- Sindhura, K.S.; Prasad, T.N.V.K.V.; Selvam, P.P.; Hussain, O.M. Synthesis, characterization and evaluation of effect of phytogenic zinc nanoparticles on soil exo-enzymes. Appl. Nanosci. 2014, 4, 819–827. [Google Scholar] [CrossRef]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta A 2015, 136, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Darroudi, M.; Sabouri, Z.; Oskuee, R.K.; Zak, A.K.; Kargar, H.; Hamid, M.H.N.A. Green chemistry approach for the synthesis of ZnO nanopowders and their cytotoxic effects. Ceram. Int. 2014, 40, 4827–4831. [Google Scholar] [CrossRef]
- Sivaraj, R.; Rahman, P.K.S.M.; Rajiv, G.P.; Venckatesh, R. Biogenic zinc oxide nanoparticles synthesis using Tabernaemontana Divaricate leaf extract and its anticancer activity against MCF-7 breast cancer cell Lines. Int. Conf. Advan. Agric. Biol. Environ. Sci. 2014, 83–85. [Google Scholar]
- Zirconium and Zirconium Compounds. Available online: http://www.gezondheidsraad.nl/sites/default/files/0015059OSH.pdf (accessed on 28 May 2015).
- Saridag, S.; Tak, O.; Alniacik, G. Basic properties and types of zirconia: An overview. World J. Stomatol. 2013, 2, 40–47. [Google Scholar]
- Li, Q.; Deacon, A.D.; Coleman, N.J. The impact of zirconium oxide nanoparticles on the hydration chemistry and biocompatibility of white Portland cement. Dent. Mater. J. 2013, 32, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Takamura, K.; Hayashi, K.; Ishinishi, N.; Yamada, T.; Sugioka, Y. Evaluation of carcinogenicity and chronic toxicity associated with orthopedic implants in mice. J. Biomed. Mater. Res. 1994, 28, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Jangra, S.L.; Stalin, L.; Dilbaghi, N.; Kumar, S.; Tawale, J.; Singh, S.P.; Pasricha, R. Antimicrobial activity of zirconia (ZrO2) nanoparticles and zirconium complexes. J. Nanosci. Nanotechnol. 2012, 12, 7105–7112. [Google Scholar] [CrossRef] [PubMed]
- Roopan, S.M.; Kumar, S.H.S.; Madhumitha, G.; Suthindhira, K. Biogenic-production of SnO2 nanoparticles and its cytotoxic effect against hepatocellular carcinoma cell line (HepG2). Appl. Biochem. Biotechnol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Husain, Q. Influence of pH and temperature on the activity of SnO2-bound alpha-amylase: A genotoxicity assessment of SnO2 nanoparticles. Prep. Biochem. Biotech. 2014, 44, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-S.; Duffin, R.; Bradley, M.; Megson, I.L.; MacNee, W.; Lee, J.K.; Jeong, J.; Donaldson, K. Predictive value of in vitro assays depends on the mechanism of toxicity of metal oxide nanoparticles. Part. Fibre Toxiol. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Buesen, R.; Landsiedel, R.; Sauer, U.G.; Wohlleben, W.; Groeters, S.; Strauss, V.; Kamp, H.; Ravenzwaay, B.V. Effects of SiO2, ZrO2, and BaSO4 nanomaterials with or without surface functionalization upon 28-day oral exposure to rats. Arch. Toxicol. 2014, 88, 1881–1960. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.-W.; An, Y.-J. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ. 2011, 409, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Dasari, T.P.; Pathakoti, K.; Hwang, H.-M. Determination of the mechanism of photoinduced toxicity of selected metal oxide nanoparticles (ZnO, CuO, Co3O4 and TiO2) to E. coli bacteria. J. Environ. Sci. 2013, 25, 882–888. [Google Scholar] [CrossRef]
- Goix, S.; Lévêque, T.; Xiong, T.-T.; Schreck, E.; Baeza-Squiban, A.; Geret, F.; Uzu, G.; Austruy, A.; Dumat, C. Environmental and health impacts of fine and ultrafine metallic particles: Assessment of threat scores. Environ. Res. 2014, 133, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.-S.; Kong, I.C. Toxic effects of nanoparticles on bioluminescence activity, seed germination, and gene mutation. Appl. Microbiol. Biotechnol. 2014, 98, 3295–3303. [Google Scholar] [CrossRef] [PubMed]
- Golinska, P.; Wypij, M.; Ingle, A.P.; Gupta, I.; Dahm, H.; Rai, M. Biogenic synthesis of metal nanoparticles from actinomycetes: Biomedical applications and cytotoxicity. Appl. Microbiol. Biotechnol. 2014, 98, 8083–8097. [Google Scholar] [CrossRef] [PubMed]
- Landsiedel, R.; Ma-Hock, L.; Kroll, A.; Hahn, D.; Schnekenburger, J.; Wiench, K.; Wohllben, W. Testing metal-oxide nanomaterials for human safety. Adv. Mater. 2010, 22, 2601–2627. [Google Scholar] [CrossRef] [PubMed]
- Darolles, C.; Sage, N.; Armengaud, J.; Malard, V. In vitro assessment of cobalt oxide particle toxicity: identifying and circumventing interference. Toxicol. In Vitro 2013, 27, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Djurisic, A.B.; Leung, Y.H.; Ng, A.M.C.; Xu, X.Y.; Lee, P.K.H.; Degger, N.; Wu, R.S.S. Toxicity of metal oxide nanoparticles: Mechanisms, characterization, and avoiding experimental artefacts. Small 2015, 11, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Adner, D.; Korb, M.; Schulze, S.; Hietschol, M.; Lang, H. A straightforward approach to oxide-free copper nanoparticles by thermal decomposition of a copper(I) precursor. Chem. Commun. 2013, 49, 6855–6857. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Jouiad, M.; Khraished, M.; Hashaikeh, R. Facile synthesis of copper oxide nanoparticles via electrospinning. J. Nanomater. 2014, 2014, 438407. [Google Scholar] [CrossRef]
- Cuevas, R.; Duran, N.; Diez, M.C.; Tortella, G.R.; Rubilar, O. Extracellular biosynthesis of copper and copper oxide nanoparticles by Stereum hirsutum, a native white-rot fungus from chilean forests. J. Nanomater. 2015, 2015, 789089. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Wang, L.; Wang, M.; Gao, F. One-pot synthesis of water-soluble superparamagnetic iron oxide nanoparticles and their MRI contrast effects in the mouse brains. Mater. Sci. Eng. C 2015, 48, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zhang, M.; Zhang, W.; Yang, L.; Wang, C.; Chen, Z. Preparation of nanocrystalline antimony oxide powders by use of gamma-ray radiation-oxidization route. Mater. Sci. Eng. B 1997, 49, 42–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Zhang, J.; Zhang, L. Shape-controlled growth of one-dimensional Sb2O3 nanomaterials. Nanotechnology 2004, 15, 762–765. [Google Scholar] [CrossRef]
- Yamada, H.; Urata, C.; Ujiie, H.; Yamauchi, Y.; Kuroda, K. Preparation of aqueous colloidal mesostructured and mesoporous silica nanoparticles with controlled particle size in a very wide range from 20 nm to 700 nm. Nanoscale 2013, 5, 6145–6153. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Yang, Y.; O´Brien, J.S.; Breznan, D.; Nimesh, S.; Bernatchez, S.; Hill, M.; Sayari, A.; Vincent, R.; Kumarathasan, P. Synthesis and physicochemical characterization of mesoporous SiO2 nanoparticles. J. Nanomater. 2014, 2014, 176015. [Google Scholar] [CrossRef]
- Arthi, G.; Archana, J.; Navaneethan, M.; Ponnusamy, S.; Hayakawa, Y.; Muthamizhchelvan, C. Solvothermal growth of diethylamine capped TiO2 nanoparticles and functional properties. J. Mater. Sci Mater. Electron 2015, 26, 2380–2383. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Eskandarloo, H. Preparation of TiO2 nanoparticles by the sol–gel method under different pH conditions and modeling of photocatalytic activity by artificial neural network. Res. Chem. Intermed. 2015, 41, 2001–2017. [Google Scholar] [CrossRef]
- Rath, M.C.; Naik, D.B. Post-irradiation induction time in the radiolytic synthesis of UO2 nanoparticles in aqueous solutions. J. Nucl. Mater. 2014, 454, 54–59. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, L.; Gu, Z.J.; Yuang, L.Y.; Xiao, C.L.; Zhao, Y.L.; Cahi, Z.F.; Shi, W.Q. A facile additive-free method for tunable fabrication of UO2 and U3O8 nanoparticles in aqueous solution. Cryst. Eng. Comm. 2014, 16, 2645–2651. [Google Scholar] [CrossRef]
- Ali, M.A.; Idris, M.R.; Quayum, M.E. Fabrication of ZnO nanoparticles by solution combustion method for the photocatalytic degradation of organic dye. J. Nanostructure Chem. 2013, 3, 36. [Google Scholar] [CrossRef]
- Bai, X.; Li, J.; Liu, H.; Tan, L.; Liu, T.; Meng, X. Solvothermal synthesis of ZnO nanoparticles and anti-infection application in vivo. ACS Appl. Mater. Interfaces 2015, 7, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Ghoul, J.E.; Kraini, M.; Mir, L.E. Synthesis of Co-doped ZnO nanoparticles by sol–gel method and its characterization. J. Mater. Sci: Mater. Electron. 2015, 26, 2555–2562. [Google Scholar] [CrossRef]
- Hajizadeh-Oghaz, M.; Razavi, R.S.; Khajelakzay, M. Optimizing sol–gel synthesis of magnesia-stabilized zirconia (MSZ) nanoparticles using Taguchi robust design for thermal barrier coatings (TBCs) applications. J. Sol-Gel Sci. Technol. 2015, 73, 227–241. [Google Scholar] [CrossRef]
- Rabjbar, M.; Lahooti, M.; Yousefi, M.; Malekzadeh, A. Sonochemical synthesis and characterization of nano-sized zirconium(IV) complex: New precursor for the preparation of pure monoclinic and tetragonal zirconia nanoparticles. J. Iran Chem. Soc. 2014, 11, 1257–1264. [Google Scholar] [CrossRef]
- Sreeremya, T.S.; Krishnan, A.; Satapathy, L.N.; Ghosh, S. Facile synthetic strategy of oleophilic zirconia nanoparticles allows preparation of highly stable thermo-conductive coolant. RSC Adv. 2014, 4, 28020–28028. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ahmaruzzaman, M.; Sinha, T. A novel approach for the synthesis of SnO2 nanoparticles and its application as a catalyst in the reduction and photodegradation of organic compounds. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 136, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Qi, F.; Zhang, S.; Li, Y.; Wang, Y.; Cao, J.; Bala, H.; Wang, X.; Jia, T.; Zhang, Z. Synthesis and enhanced gas sensing properties of flower-like SnO2 hierarchical structures decorated with discrete ZnO nanoparticles. J. Alloys Compd. 2014, 25, 192–199. [Google Scholar] [CrossRef]
- Zamand, N.; Pour, A.N.; Housaindokht, M.R.; Izadyar, M. Size-controlled synthesis of SnO2 nanoparticles using reverse microemulsion method. Solid State Sci. 2014, 33, 6–11. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seabra, A.B.; Durán, N. Nanotoxicology of Metal Oxide Nanoparticles. Metals 2015, 5, 934-975. https://doi.org/10.3390/met5020934
Seabra AB, Durán N. Nanotoxicology of Metal Oxide Nanoparticles. Metals. 2015; 5(2):934-975. https://doi.org/10.3390/met5020934
Chicago/Turabian StyleSeabra, Amedea B., and Nelson Durán. 2015. "Nanotoxicology of Metal Oxide Nanoparticles" Metals 5, no. 2: 934-975. https://doi.org/10.3390/met5020934