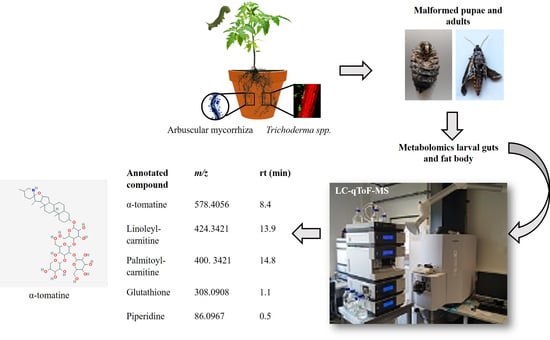
Cascading Effects of Root Microbial Symbiosis on the Development and Metabolome of the Insect Herbivore Manduca sexta L.
Abstract
:1. Introduction
2. Results
2.1. Root Colonization by Rhizophagus irregularis or Trichoderma harzianum Impaired Pupation and Adult Emergence of Manduca sexta Larvae
2.2. Root Colonization by Rhizophagus irregularis or Trichoderma harzianum Altered the Metabolomic Profile of Tomato Leaves
2.3. Root Colonization by Rhizophagus irregularis or Trichoderma harzianum Altered the Metabolic Profile of the Manduca sexta Larval Gut
2.4. Root Colonization by Rhizophagus irregularis or Trichoderma harzianum Altered the Metabolic Profile of the Manduca sexta Larval Fat Body
3. Discussion
4. Materials and Methods
4.1. Plant, Fungal, and Insect Material
4.2. Plant Growth Conditions and Fungal Inoculation
4.3. Rhizophagus irregularis and Trichoderma harzianum Root Colonization
4.4. Bioassay for the Assessment of the Impact of Rhizophagus irregularis and Trichoderma harzianum on Manduca sexta Pupation and Adult Emergence
4.5. Bioassay for the Assessment of the Impact of Rhizophagus irregularis and Trichoderma harzianum on Tomato Leaf Metabolome
4.6. Bioassay to Assess the Impact of Rhizophagus irregularis and Trichoderma harzianum on the Metabolome of Manduca sexta Gut and Fat Body
4.7. Leaf Metabolomics
4.7.1. Extraction of Leaf Metabolites
4.7.2. Liquid Chromatography–Quadrupole Time of Flight–Mass Spectrometry and Data Analysis of the Leaf Extracts
4.8. Dissection of Manduca sexta Larvae and Separation of the Gut and Fat Body Samples
4.9. Insect Metabolomics
4.9.1. Extraction of Metabolites from the Gut and Fat Body of Manduca sexta
4.9.2. Ultra High Performance Liquid Chromatography–Quadrupole Time of Flight–Mass Spectrometry and Data Analysis of the Insect Extracts
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furstenberg-Hagg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karban, R.; Baldwin, I. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Feeny, P.; Rosenthal, G.; Berenbaum, M. The evolution of chemical ecology: Contributions from the study of herbivorous insects. Herbiv. Interact. Second. Plant Metab. 1992, 2, 1–44. [Google Scholar]
- Falco, M.C.; Marbach, P.A.S.; Pompermayer, P.; Lopes, F.C.C.; Silva-Filho, M.C. Mechanisms of sugarcane response to herbivory. Genet. Mol. Biol. 2001, 24, 113–122. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Protect. Sci. 2016, 52, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Pavela, R.; Maggi, F.; Lupidi, G.; Cianfaglione, K.; Dauvergne, X.; Bruno, M.; Benelli, G. Efficacy of sea fennel (Crithmum maritimum L., Apiaceae) essential oils against culex quinquefasciatus say and spodoptera littoralis (Boisd.). Ind. Crop. Prod. 2017, 109, 603–610. [Google Scholar] [CrossRef]
- Bloomquist, J.; Boina, D.; Chow, E.; Carlier, P.; Reina, M.; Gonzalez-Coloma, A. Mode of action of the plant-derived silphinenes on insect and mammalian GABAA receptor/chloride channel complex. Pestic. Biochem. Physiol. 2008, 91, 17–23. [Google Scholar] [CrossRef]
- Mbata, G.N.; Payton, M.E. Effect of monoterpenoids on oviposition and mortality of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) under hermetic conditions. J. Stored Prod. Res. 2013, 53, 43–47. [Google Scholar] [CrossRef]
- Yeom, H.-J.; Kang, J.S.; Kim, G.-H.; Park, I.-K. Insecticidal and acetylcholine esterase inhibition activity of Apiaceae plant essential oils and their constituents against adults of German cockroach (Blattella germanica). J. Agric. Food Chem. 2012, 60, 7194–7203. [Google Scholar] [CrossRef]
- Langenheim, J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef]
- Zagrobelny, M.; Bak, S.; Rasmussen, A.V.; Jørgensen, B.; Naumann, C.M.; Møller, B.L. Cyanogenic glucosides and plant–insect interactions. Phytochemistry 2004, 65, 293–306. [Google Scholar] [CrossRef]
- Willinger, G.; Dobler, S. Selective sequestration of iridoid glycosides from their host plants in longitarsus flea beetles. Biochem. Syst. Ecol. 2001, 29, 335–346. [Google Scholar] [CrossRef]
- Nishida, R. Sequestration of defensive substances from plants by lepidoptera. Annu. Rev. Entomol. 2002, 47, 57–92. [Google Scholar] [CrossRef]
- Ode, P.J. Plant chemistry and natural enemy fitness: Effects on herbivore and natural enemy interactions. Annu. Rev. Entomol. 2006, 51, 163–185. [Google Scholar] [CrossRef] [Green Version]
- Mendes, R.; Raaijmakers, J.M. Cross-kingdom similarities in microbiome functions. ISME J. 2015, 9, 1905–1907. [Google Scholar] [CrossRef] [Green Version]
- Van Der Heijden, M.G.A.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiu, Y.-L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant—Fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codon, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Martinez-Medina, A.; Pozo, M.J.; Cammue, B.P.; Vos, C.M. Belowground defence strategies in plants: The plant–Trichoderma dialogue. In Belowground Defence Strategies in Plants; Springer: Berlin/Heidelberg, Germany, 2016; pp. 301–327. [Google Scholar]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Bowles, T.M.; Barrios-Masias, F.H.; Carlisle, E.A.; Cavagnaro, T.R.; Jackson, L.E. Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci. Total Environ. 2016, 566, 1223–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E. The relationship between increased growth and resistance induced in plants by root colonizing microbes. Plant Signal. Behav. 2008, 3, 737–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [Green Version]
- Goverde, M.; van der Heijden, M.V.D.H.; Wiemken, A.; Sanders, I.; Erhardt, A. Arbuscular mycorrhizal fungi influence life history traits of a lepidopteran herbivore. Oecologia 2000, 125, 362–369. [Google Scholar] [CrossRef]
- Pineda, A.; Dicke, M.; Pieterse, C.M.J.; Pozo, M.J. Beneficial microbes in a changing environment: Are they always helping plants to deal with insects? Funct. Ecol. 2013, 27, 574–586. [Google Scholar] [CrossRef] [Green Version]
- Schoonhoven, L.; Van Loon, B.; van Loon, J.; Dicke, M. Insect-Plant Biology; Oxford University Press on Demand: Oxford, UK, 2005. [Google Scholar]
- Gange, A.C.; West, H.M. Interactions between arbuscular mycorrhizal fungi and foliar-feeding insects in Plantago lanceolata L. New Phytol. 1994, 128, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Larose, G.; Chênevert, R.; Moutoglis, P.; Gagné, S.; Piché, Y.; Vierheilig, H. Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. J. Plant Physiol. 2002, 159, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- Hill, E.M.; Robinson, L.A.; Abdul-Sada, A.; Vanbergen, A.J.; Hodge, A.; Hartley, S.E. Arbuscular mycorrhizal fungi and plant chemical defence: Effects of colonisation on aboveground and belowground metabolomes. J. Chem. Ecol. 2018, 44, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.C.; Jin, W.J.; Liu, A.R.; Zhang, S.J.; Liu, D.L.; Wang, F.H.; Lin, X.M.; He, C.X. Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperature stress. Sci. Hortic. 2013, 160, 222–229. [Google Scholar] [CrossRef]
- Rivero, J.; Lidoy, J.; Llopis-Giménez, Á.; Herrero, S.; Flors, V.; Pozo, M.J. Mycorrhizal symbiosis primes the accumulation of antiherbivore compounds and enhances herbivore mortality in tomato. J. Exp. Bot. 2021, 72, 5038–5050. [Google Scholar] [CrossRef]
- Coppola, M.; Diretto, G.; Digilio, M.C.; Woo, S.L.; Giuliano, G.; Molisso, D.; Pennacchio, F.; Lorito, M.; Rao, R. Transcriptome and metabolome reprogramming in tomato plants by Trichoderma harzianum strain T22 primes and enhances defense responses against aphids. Front. Physiol. 2019, 10, 745. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Yedidia, I.; Chet, I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 2005, 95, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y.; Chet, I. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Medina, A.; Roldán, A.; Albacete, A.; Pascual, J.A. The interaction with arbuscular mycorrhizal fungi or Trichoderma harzianum alters the shoot hormonal profile in melon plants. Phytochemistry 2011, 72, 223–229. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Alguacil, M.D.M.; Pascual, J.A.; Van Wees, S.C. Phytohormone profiles induced by Trichoderma isolates correspond with their biocontrol and plant growth-promoting activity on melon plants. J. Chem. Ecol. 2014, 40, 804–815. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, X.F.; Guo, J.; dos-Santos, M.L.; Vivanco, J.M. Trichoderma gamsii affected herbivore feeding behaviour on Arabidopsis thaliana by modifying the leaf metabolome and phytohormones. Microb. Biotechnol. 2018, 11, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Koricheva, J.; Gange, A.C.; Jones, T. Effects of mycorrhizal fungi on insect herbivores: A meta-analysis. Ecology 2009, 90, 2088–2097. [Google Scholar] [CrossRef]
- Malinowska, J.M.; Viant, M.R. Confidence in metabolite identification dictates the applicability of metabolomics to regulatory toxicology. Curr. Opin. Toxicol. 2019, 16, 32–38. [Google Scholar] [CrossRef]
- Kariyat, R.R.; Mauck, K.E.; Balogh, C.M.; Stephenson, A.G.; Mescher, M.C.; De Moraes, C.M. Inbreeding in horsenettle (Solanum carolinense) alters night-time volatile emissions that guide oviposition by Manduca sexta moths. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130020. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.G.; Philogène, B.J.R. Reproductive potential of laboratory-reared Manduca sexta (Lepidoptera: Sphingidae) as affected by sex ratio. Can. Entomol. 1983, 115, 295–298. [Google Scholar] [CrossRef]
- Tikkanen, O.P.; Niemelä, P.; Keränen, J. Growth and development of a generalist insect herbivore, Operophtera brumata, on original and alternative host plants. Oecologia 2000, 122, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Teder, T.; Tammaru, T. Sexual size dimorphism within species increases with body size in insects. Oikos 2005, 108, 321–334. [Google Scholar] [CrossRef]
- Bell, R.A. Insect Neurochemistry and Neurophysiology 1986; Springer: Berlin/Heidelberg, Germany, 1986; pp. 347–350. [Google Scholar]
- West, S.A.; Sheldon, B.C. Constraints in the evolution of sex ratio adjustment. Science 2002, 295, 1685–1688. [Google Scholar] [CrossRef] [Green Version]
- Kaling, M.; Schmidt, A.; Moritz, F.; Rosenkranz, M.; Witting, M.; Kasper, K.; Janz, D.; Schmitt-Kopplin, P.; Schnitzler, J.P.; Polle, A. Mycorrhiza-triggered transcriptomic and metabolomic networks impinge on herbivore fitness. Plant Physiol. 2018, 176, 2639–2656. [Google Scholar] [CrossRef] [Green Version]
- Hare, J.D. Seasonal variation in plant-insect associations: Utilization of Solanum dulcamara by Leptinotarsa decemlineata. Ecology 1983, 64, 345–361. [Google Scholar] [CrossRef]
- Altesor, P.; García, Á.; Font, E.; Rodríguez-Haralambides, A.; Vilaró, F.; Oesterheld, M.; Soler, R.; González, A. Glycoalkaloids of wild and cultivated Solanum: Effects on specialist and generalist insect herbivores. J. Chem. Ecol. 2014, 40, 599–608. [Google Scholar] [CrossRef]
- Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F. A review of bioinsecticidal activity of Solanaceae alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Ortiz, E.V.; Garrido, E.; Poveda, K.; Jander, G. Potato tuber herbivory increases resistance to aboveground lepidopteran herbivores. Oecologia 2016, 182, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Weissenberg, M.; Levy, A.; Svoboda, J.A.; Ishaaya, I. The effect of some Solanum steroidal alkaloids and glycoalkaloids on larvae of the red flour beetle, Tribolium castaneum, and the tobacco hornworm, Manduca sexta. Phytochemistry 1998, 47, 203–209. [Google Scholar] [CrossRef]
- Duffey, S.S.; Bloem, K.A.; Campbell, B.C. Consequences of Sequestration of Plant Natural Products Inplant-Insect-Parasitoid Interactions. John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Friedman, M. Tomato glycoalkaloids: Role in the plant and in the diet. J. Agric. Food Chem. 2002, 50, 5751–5780. [Google Scholar] [CrossRef]
- Büyükgüzel, E.; Büyükgüzel, K.; Erdem, M.; Adamski, Z.; Adamski, Z.; Marciniak, P.; Ziemnicki, K.; Ventrella, E.; Scrano, L.; Bufo, S.A. The influence of dietary α-solanine on the waxmoth Galleria mellonella L. Arch. Insect Biochem. Physiol. 2013, 83, 15–24. [Google Scholar] [CrossRef]
- Adamski, Z.; Adamski, Z.; Marciniak, P.; Ziemnicki, K.; Büyükgüzel, E.; Erdem, M.; Büyükgüzel, K.; Ventrella, E.; Falabella, P.; Cristallo, M. Potato leaf extract and its component, α-solanine, exert similar impacts on development and oxidative stress in galleria mellonella L. Arch. Insect Biochem. Physiol. 2014, 87, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Flanders, K.L.; Hawkes, J.G.; Radcliffe, E.B.; Lauer, F.I. Insect resistance in potatoes: Sources, evolutionary relationships, morphological and chemical defenses, and ecogeographical associations. Euphytica 1992, 61, 83–111. [Google Scholar] [CrossRef]
- Pelletier, Y.; Tai, G.C.C. Genotypic variability and mode of action of Colorado potato beetle (Coleoptera: Chrysomelidae) resistance in seven Solanum species. J. Econ. Entom. 2001, 94, 572–578. [Google Scholar] [CrossRef] [PubMed]
- López-Carretero, A.; Cruz, M.; Eben, A. Phenotypic plasticity of the reproductive system of female Leptinotarsa undecimlineata. Entomol. Exp. Appl. 2005, 115, 27–31. [Google Scholar] [CrossRef]
- Keukens, E.A.J.; de Vrije, T.; van den Boom, C.; de Waard, P.; Plasman, H.H.; Thiel, F.; Chupin, V.; Jongen, W.M.F.; de Kruijff, B. Molecular basis of glycoalkaloid induced membrane disruption. Biochim. Biophys. Acta BBA-Biomembr. 1995, 1240, 216–228. [Google Scholar] [CrossRef] [Green Version]
- Oberdörster, E.; Clay, M.A.; Cottam, D.M.; Wilmot, F.A.; McLachlan, J.A.; Milner, M.J. Common phytochemicals are ecdysteroid agonists and antagonists: A possible evolutionary link between vertebrate and invertebrate steroid hormones. J. Steroid Biochem. Mol. Biol. 2001, 77, 229–238. [Google Scholar] [CrossRef]
- Morris, C.E. Electrophysiological effects of cholinergic agents on the CNS of a nicotine-resistant insect, the tobacco hornworm (Manduca sexta). J. Exp. Zool. 1984, 229, 361–374. [Google Scholar] [CrossRef]
- Wink, M.; Theile, V. Alkaloid tolerance in Manduca sexta and phylogenetically related sphingids (Lepidoptera: Sphingidae). Chemoecology 2002, 12, 29–46. [Google Scholar] [CrossRef]
- Maddrell, S.H.; Gardiner, B.O. Induction of transport of organic anions in Malpighian tubules of Rhodnius. J. Exp. Biol. 1975, 63, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, V.D.A.; da Silva, A.M.M.; Silva, J.H.C.E.; Costa, S.L. Neurotoxicity of Prosopis juliflora: From natural poisoning to mechanism of action of its piperidine alkaloids. Neurotox. Res 2018, 34, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.I.; Nawaz, S.A.; Azim, M.K.; Ghayur, M.N.; Lodhi, M.A.; Jalil, S.; Khalid, A.; Ahmed, A.; Rode, B.M.; Ahmad, V.U. Juliflorine: A potent natural peripheral anionic-site-binding inhibitor of acetylcholinesterase with calcium-channel blocking potential, a leading candidate for Alzheimer’s disease therapy. Biochem. Biophys. Res. Commun. 2005, 332, 1171–1179. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Perez-Rodriguez, V.; Souders II, C.L.; Martyniuk, C.J. Assessing the toxicity of the benzamide fungicide zoxamide in zebrafish (Danio rerio): Towards an adverse outcome pathway for beta-tubulin inhibitors. Environ. Toxicol. Pharmacol. 2020, 78, 103405. [Google Scholar] [CrossRef]
- Nwani, C.D.; Odo, G.E.; Nwadinigwe, A.O.; Onyeke, C.C.; Atama, C.I.; Ngwu, G.; Oluah, S.N.; Ukonze, J.A.; Ezeibe, B.C.A. Short-term effects of albendazole on the oxidative stress markers and hematological parameters in tissues of African Catfish Clarias gariepinus. J. Aquat. Anim. Health 2016, 28, 222–228. [Google Scholar] [CrossRef]
- Andrade, T.S.; Henriques, J.F.; Almeida, A.R.; Machado, A.L.; Koba, O.; Giang, P.T.; Soares, A.M.; Domingues, I. Carbendazim exposure induces developmental, biochemical and behavioural disturbance in zebrafish embryos. Aquat. Toxicol. 2016, 170, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lait, C.G.; Alborn, H.T.; Teal, P.E.A.; Tumlinson, J.H. Rapid biosynthesis of N-linolenoyl-L-glutamine, an elicitor of plant volatiles, by membrane-associated enzyme (s) in Manduca sexta. Proc. Natl. Acad. Sci. USA 2003, 100, 7027–7032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshinaga, N.; Aboshi, T.; Abe, H.; Nishida, R.; Alborn, H.T.; Tumlinson, J.H.; Mori, N. Active role of fatty acid amino acid conjugates in nitrogen metabolism in Spodoptera litura larvae. Proc. Natl. Acad. Sci. USA 2008, 105, 18058–18063. [Google Scholar] [CrossRef] [Green Version]
- Yoshinaga, N.; Ishikawa, C.; Seidl-Adams, I.; Bosak, E.; Aboshi, T.; Tumlinson, J.H.; Mori, N. N-(18-hydroxylinolenoyl)-L-glutamine: A newly discovered analog of volicitin in Manduca sexta and its elicitor activity in plants. J. Chem. Ecol. 2014, 40, 484–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Block, A.; Christensen, S.A.; Hunter, C.T.; Alborn, H.T. Herbivore-derived fatty-acid amides elicit reactive oxygen species burst in plants. J. Exp. Bot. 2018, 69, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Paré, P.W.; Alborn, H.T.; Tumlinson, J.H. Concerted biosynthesis of an insect elicitor of plant volatiles. Proc. Natl. Acad. Sci. USA 1998, 95, 13971–13975. [Google Scholar] [CrossRef] [Green Version]
- Tumlinson, J.H.; Lait, C.G. Biosynthesis of fatty acid amide elicitors of plant volatiles by insect herbivores. Arch. Insect Biochem. Physiol. Collab. Entomol. Soc. Am. 2005, 58, 54–68. [Google Scholar] [CrossRef]
- Mori, N.; Yoshinaga, N. Function and evolutionary diversity of fatty acid amino acid conjugates in insects. J. Plant Interact. 2011, 6, 103–107. [Google Scholar] [CrossRef]
- Horne, I.; Haritos, V.S.; Oakeshott, J.G. Comparative and functional genomics of lipases in holometabolous insects. Insect Biochem. Mol. Biol. 2009, 39, 547–567. [Google Scholar] [CrossRef] [PubMed]
- Stanley-Samuelson, D.W.; Jurenka, R.A.; Cripps, C.; Blomquist, G.J.; de Renobales, M. Fatty acids in insects: Composition, metabolism, and biological significance. Arch. Insect Biochem. Physiol. 1988, 9, 1–33. [Google Scholar] [CrossRef]
- Soulages, J.; Wells, M.A. Metabolic fate and turnover rate of hemolymph free fatty acids in adult Manduca sexta. Insect Biochem. Mol. Biol. 1994, 24, 79–86. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Wrońska, A.K.; Kazek, M.; Boguś, M.I. Metamorphosis-related changes in the free fatty acid profiles of Sarcophaga (Liopygia) argyrostoma (Robineau-Desvoidy, 1830). Sci. Rep. 2020, 10, 17337. [Google Scholar] [CrossRef] [PubMed]
- Bremer, J. Carnitine-metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480. [Google Scholar] [CrossRef] [PubMed]
- Liepinsh, E.; Kalvinsh, I.; Dambrova, M. The regulation of energy metabolism pathways through L-carnitine homeostasis. Role Adipocyte Dev. Type 2 Diabetes 2011, 6, 107–128. [Google Scholar]
- Li, J.-M.; Li, L.-Y.; Qin, X.; Ning, L.-J.; Lu, D.-L.; Li, D.-L.; Zhang, M.-L.; Wang, X.; Du, Z.-Y. Systemic regulation of L-carnitine in nutritional metabolism in zebrafish, Danio rerio. Sci. Rep. 2017, 7, 40815. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Guo, W.; Yang, L.; He, Q.; Zhou, S. Juvenile hormone promotes locust fat body cell polyploidization and vitellogenesis by activating the transcription of Cdk6 and E2f1. Insect Biochem. Mol. Biol. 2018, 102, 1–10. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Roldán, A.; Pascual, J.A. Performance of a Trichoderma harzianum bentonite—Vermiculite formulation against fusarium wilt in seedling nursery melon plants. Hortic. Sci. 2009, 44, 2025–2027. [Google Scholar] [CrossRef] [Green Version]
- Grosse-Wilde, E.; Kuebler, L.S.; Bucks, S.; Vogel, H.; Wicher, D.; Hansson, B.S. Antennal transcriptome of Manduca sexta. Proc. Natl. Acad. Sci. USA 2011, 108, 7449–7454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, M.P.; Quast, V.; Van Dam, N.M.; Eisenhauer, N.; Roscher, C.; Biere, A.; Martinez-Medina, A. Interactions between functionally diverse fungal mutualists inconsistently affect plant performance and competition. Oikos 2019, 128, 1136–1146. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. In Circular; California Agricultural Experiment Station: Berkeley, CA, USA, 1950; Volume 347, p. 32. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Rogachev, I.; Aharoni, A. UPLC-MS-based metabolite analysis in tomato. In Plant Metabolomics; Springer: Berlin/Heidelberg, Germany, 2011; pp. 129–144. [Google Scholar]
- De Vos, R.; Schipper, B.; Hall, R. High-performance liquid chromatography–mass spectrometry analysis of plant metabolites in Brassicaceae. In Plant Metabolomics; Springer: Berlin/Heidelberg, Germany, 2011; pp. 111–128. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]






| Treatment | No Larvae Reaching the Pre-Pupa Stage | Days Until Pre-Pupation (±SD) | Days until Pupation (±SD) | Total No of Pupae | No. of Pupae with Normal Morphology | No. of Pupae with Anomalous Morphology | Weight (g) of Normal Pupae (±SD) | F/M Ratio of the Normal Pupae | Number of Normal Pupae Not Eclosing | No of Moths with Anomalous Morphology | No of Moths with Normal Morphology |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-inoculated | 19 | 23.39 ± 1.47 | 22.06 ± 3.83 | 17 | 13 | 4 | 2.65 ± 0.49 | 9:4 | 2 | 1 | 10 |
| R. irregularis | 15 | 20.41 ± 2.54 | 19.94 ± 4.45 | 15 | 11 | 4 | 2.71 ± 0.61 | 3:8 | 3 | 4 | 4 |
| T. harzianum | 13 | 22.69 ± 1.9 | 11.61 ± 6.59 | 12 | 5 | 7 | 1.61 ± 0.91 | 2:3 | 1 | 2 | 2 |
| One-way ANOVA | - | ns | ns | - | - | - | ns | χ2 = 4.196 | - | - | - |
| Feature ID | Annotation | m/z | rt | FC | log2(FC) | Raw p-Value | Level of Confidence |
|---|---|---|---|---|---|---|---|
| 1 | C23H39NO4 | 394.2951 | 12.0 | 5.63 | 2.5 | p < 0.0001 | 3 |
| 2 | C10H17N3O6S (Glutathione) | 308.0908 | 1.1 | 5.61 | 2.49 | p = 0.0006 | 2 |
| 3 | C24H41NO3 | 392.3157 | 17.9 | 6.25 | 2.64 | p = 0.002 | 3 |
| 4 | C23H39NO3 | 378.3012 | 17.4 | 6.56 | 2.71 | p = 0.01 | 3 |
| 5 | C27H39O3 | 426.3009 | 17.9 | 6.49 | 2.7 | p = 0.04 | 3 |
| 6 | C5H11N (Piperidine) | 86.0967 | 0.5 | 0.49 | −1.03 | p < 0.0001 | 2 |
| 7 | C55H94N10O16P2 | 623.3105 | 8.0 | 0.37 | −1.45 | p < 0.0001 | 3 |
| 8 | C52H97N8O17P3S | 616.3028 | 8.0 | 0.36 | −1.48 | p < 0.0001 | 3 |
| 9 | No formula | 110.0653 | 1.2 | 0.28 | −1.84 | p < 0.0001 | 4 |
| 10 | No formula | 132.1018 | 0.4 | 0.49 | −1.03 | p < 0.0001 | 4 |
| 11 | C12H37N7OS | 328.2841 | 13.1 | 0.02 | −6.05 | p < 0.0001 | 3 |
| 12 | C23H43N3O6P2 | 520.2708 | 14.9 | 0.02 | −5.76 | p < 0.0001 | 3 |
| 13 | C14H18N2O5 | 295.1293 | 4.9 | 0.004 | −8.16 | p = 0.002 | 3 |
| 14 | No formula | 782.0187 | 8.2 | 0.03 | −5.1 | p = 0.001 | 4 |
| 15 | No formula | 644.2370 | 10.4 | 0.03 | −4.5 | p = 0.003 | 4 |
| Feature ID | Annotation | m/z | rt | FC | log2(FC) | Raw p-Value | Level of Confidence |
|---|---|---|---|---|---|---|---|
| 1 | C23H39NO4 | 394.2951 | 12.0 | 378.82 | 8.57 | p < 0.0001 | 3 |
| 2 | C25H45NO4 (linoleyl-carnitine) | 424.3421 | 13.9 | 597.13 | 9.22 | p < 0.0001 | 2 |
| 3 | C22H42N2O | 351.3370 | 12.9 | 614.71 | 9.26 | p< 0.0001 | 3 |
| 4 | No formula | 224.2010 | 9.8 | 388.27 | 8.6 | p < 0.0001 | 4 |
| 5 | C25H43NO4 (γ-linolenoyl-carnitine) | 422.3274 | 13.2 | 340.61 | 8.41 | p < 0.0001 | 2 |
| 6 | C23H42NO7P | 476.2772 | 13.0 | 0.19 | −2.4 | p < 0.0001 | 3 |
| 7 | C13H16N4 | 229.1444 | 7.9 | 0.1 | −3.31 | p < 0.0001 | 3 |
| 8 | No formula | 110.0653 | 1.2 | 0.2 | −2.34 | p < 0.0001 | 4 |
| 9 | C8H9NO2 | 152.0709 | 6.7 | 0.05 | −4.34 | p < 0.0001 | 3 |
| 10 | C10H9NO4 | 225.0872 | 1.3 | 0.16 | −2.67 | p < 0.0001 | 3 |
| 11 | No formula | 133.0866 | 7.9 | 0.01 | −6.13 | p < 0.0001 | 4 |
| 12 | No formula | 812.0668 | 8.0 | 0.009 | −6.84 | p < 0.0001 | 4 |
| 13 | C12H37N7OS | 328.2841 | 13.1 | 0.01 | −6.42 | p < 0.0001 | 3 |
| 14 | C23H45NO4(O-palmitoyl-carnitine) | 400.3421 | 14.8 | 0.01 | −6.31 | p < 0.0001 | 2 |
| 15 | C14H18N2O5 | 295.1293 | 4.9 | 0.005 | −7.74 | 0.0001 | 3 |
| Feature ID | Annotation | m/z | rt | FC | log2(FC) | p-Value | Level of Confidence |
|---|---|---|---|---|---|---|---|
| 1 | C25H43NO5 | 438.3210 | 11.9 | 3.71 | 1.89 | p < 0.0001 | 3 |
| 2 | C32H66N4O7P2S | 357.2138 | 6.8 | 3.52 | 1.82 | p < 0.0001 | 3 |
| 3 | C10H12N2O3 | 209.0909 | 0.9 | 4.73 | 2.24 | p < 0.0001 | 3 |
| 4 | C37H62N6 | 591.5095 | 15.1 | 3.29 | 1.72 | p < 0.0001 | 3 |
| 5 | C36H74N2O2 | 567.5823 | 19.9 | 3.64 | 1.87 | p < 0.008 | 3 |
| 6 | C23H4NO7P | 476.2774 | 11.7 | 0.4 | −1.31 | p < 0.0001 | 3 |
| 7 | No formula | 175.1163 | 1.1 | 0.37 | −1.42 | p < 0.0001 | 4 |
| 8 | No formula | 112.0502 | 1.0 | 0.28 | −1.82 | p < 0.0001 | 4 |
| 9 | No formula | 671.3542 | 5.9 | 0.14 | −2.83 | p < 0.0001 | 4 |
| 10 | C23H43NO4 (2E)-hexadecenoylcarnitine) | 398.3265 | 13.7 | 0.15 | −2.77 | p < 0.0001 | 2 |
| 11 | No formula | 224.2010 | 10.0 | 0.05 | −4.42 | p < 0.0001 | 4 |
| 12 | C37H66N8O10 | 392.2516 | 6.9 | 0.03 | −4.86 | p < 0.0001 | 3 |
| 13 | C50H94N8O17 | 360.5650 | 6.2 | 0.04 | −4.53 | p < 0.0001 | 3 |
| 14 | C41H79N14O5PS | 304.5352 | 5.9 | 0.04 | −4.6 | p < 0.0001 | 3 |
| 15 | C12H37N7OS | 328.2839 | 13.1 | 0.04 | −4.66 | p < 0.0001 | 3 |
| Feature ID | Annotation | m/z | rt | FC | log2(FC) | p-Value | Level of Confidence |
|---|---|---|---|---|---|---|---|
| 1 | C9H18O2 | 159.1377 | 12.9 | 6.8 | 2.77 | p < 0.0001 | 3 |
| 2 | No formula | 320.2558 | 14.5 | 12.36 | 3.63 | p < 0.0001 | 4 |
| 3 | C17H31NO | 266.2483 | 13.6 | 10.71 | 3.42 | p < 0.0001 | 3 |
| 4 | C18H37NO (octadecanamide) | 284.295 | 19.6 | 19.24 | 4.27 | p < 0.0001 | 2 |
| 5 | C36H70N2O4 | 595.5408 | 15.8 | 65.57 | 6.04 | p < 0.0001 | 3 |
| 6 | C27H50N2O2 | 435.3944 | 17.8 | 105.25 | 6.72 | p < 0.0001 | 3 |
| 7 | C36H70N2O6 | 627.5310 | 14.9 | 105.4 | 6.72 | p < 0.0001 | 3 |
| 8 | C28H50N4O8S | 603.3416 | 10.4 | 119.75 | 6.9 | p < 0.0001 | 3 |
| 9 | C15H41N6O3PS | 417.2771 | 10.6 | 62.57 | 5.97 | p < 0.0001 | 3 |
| 10 | C23H42NO7P | 476.2774 | 11.7 | 0.21 | −2.24 | p < 0.0001 | 3 |
| 11 | C44H74N5O10P | 432.7648 | 8.0 | 0.01 | −6.32 | p < 0.0001 | 3 |
| 12 | C8H16O2 (octanoic acid) | 145.1222 | 8.0 | 0.01 | −6.37 | p < 0.0001 | 2 |
| 13 | C58H77N31O7 | 660.8389 | 8.0 | 0.008 | −6.93 | p < 0.0001 | 3 |
| 14 | C35H36N4O7 (benzamide) | 625.2662 | 15.9 | 0.01 | −6.26 | p < 0.0001 | 2 |
| 15 | No formula | 742.0044 | 7.87 | 0.009 | −6.73 | p < 0.0001 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papantoniou, D.; Vergara, F.; Weinhold, A.; Quijano, T.; Khakimov, B.; Pattison, D.I.; Bak, S.; van Dam, N.M.; Martínez-Medina, A. Cascading Effects of Root Microbial Symbiosis on the Development and Metabolome of the Insect Herbivore Manduca sexta L. Metabolites 2021, 11, 731. https://doi.org/10.3390/metabo11110731
Papantoniou D, Vergara F, Weinhold A, Quijano T, Khakimov B, Pattison DI, Bak S, van Dam NM, Martínez-Medina A. Cascading Effects of Root Microbial Symbiosis on the Development and Metabolome of the Insect Herbivore Manduca sexta L. Metabolites. 2021; 11(11):731. https://doi.org/10.3390/metabo11110731
Chicago/Turabian StylePapantoniou, Dimitra, Fredd Vergara, Alexander Weinhold, Teresa Quijano, Bekzod Khakimov, David I. Pattison, Søren Bak, Nicole M. van Dam, and Ainhoa Martínez-Medina. 2021. "Cascading Effects of Root Microbial Symbiosis on the Development and Metabolome of the Insect Herbivore Manduca sexta L." Metabolites 11, no. 11: 731. https://doi.org/10.3390/metabo11110731