Design of a Novel Axial Gas Pulses Micromixer and Simulations of its Mixing Abilities via Computational Fluid Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of the Microfluidic Chip
2.1.1. Technical Constraints and Objectives
2.1.2. Strategy used to Design and Define the Mixing Microchip Pattern and Size
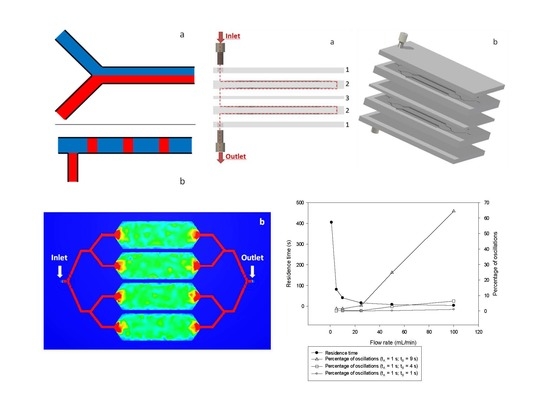
- -
- 5 mm between the inlet/outlet and the edges of the chip, to leave space for the fluidic connectors;
- -
- 8 mm for every flow division: 4 mm for splitting and 4 mm for redirecting the flow into the right direction;
- -
- 3 mm between channels and the walls of the chip;
- -
- 1 mm between channels.
2.1.3. Elaboration of the Multi-Stage Micromixer
2.2. Methodology for Simulation of the Gas Flow and Mixing
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robbins, P.A.; Swanson, G.D.; Micco, A.J.; Schubert, W.P. A fast gas-mixing system for breath-to-breath respiratory control studies. J. Appl. Physiol. Respir. Environ. Exerc Physiol. 1982, 52, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- Dantas, H.V.; Barbosa, M.F.; Moreira, P.N.T.; Galvão, R.K.H.; Araújo, M.C.U. An automatic system for accurate preparation of gas mixtures. Microchem. J. 2015, 119, 123–127. [Google Scholar] [CrossRef]
- Continuous Flow Type Gas Blending Facility Used for Autonomous and System Diving—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1876610217311748 (accessed on 17 December 2018).
- Fletcher, G.C.; Summers, G.; Corrigan, V.K.; Johanson, M.R.; Hedderley, D. Optimizing Gas Mixtures for Modified Atmosphere Packaging of Fresh King Salmon (Oncorhynchus tshawytscha). J. Aquat. Food Prod. Technol. 2005, 13, 5–28. [Google Scholar] [CrossRef]
- Hood, M.E. Gas Mixing Device for Draught Beer Dispensing. U.S. Patent 2,569,378, 25 Spetember 1951. [Google Scholar]
- Mvola, B.; Kah, P. Effects of shielding gas control: Welded joint properties in GMAW process optimization. Int. J. Adv. Manuf. Technol. 2017, 88, 2369–2387. [Google Scholar] [CrossRef]
- Shmelev, V.M.; Nikolaev, V. Propane conversion in a chemical compression reactor. Russ. J. Phys. Chem. B 2011, 5, 235–243. [Google Scholar] [CrossRef]
- Zethræus, B.; Adams, C.; Berge, N. A simple model for turbulent gas mixing in CFB reactors. Powder Technol. 1992, 69, 101–105. [Google Scholar] [CrossRef]
- Christensen, P.L.; Nielsen, J.; Kann, T. Methods to produce calibration mixtures for anesthetic gas monitors and how to perform volumetric calculations on anesthetic gases. J. Clin. Monit. Comput. 1992, 8, 279–284. [Google Scholar] [CrossRef]
- Martin, N.A.; Goody, B.A.; Wang, J.; Milton, M.J.T. Accurate and adjustable calibration gas flow by switching permeation and diffusion devices. Meas. Sci. Technol. 2012, 23, 105005. [Google Scholar] [CrossRef]
- Rosenberg, E.; Hallama, R.A.; Grasserbauer, M. Development and evaluation of a calibration gas generator for the analysis of volatile organic compounds in air based on the injection method. Fresenius J. Anal. Chem 2001, 371, 798–805. [Google Scholar] [CrossRef]
- Monsé, C.; Broding, H.; Hoffmeyer, F.; Jettkant, B.; Berresheim, H.; Brüning, T.; Bünger, J.; Sucker, K. Use of a Calibration Gas Generator for Irritation Threshold Assessment and As Supplement of Dynamic Dilution Olfactometry. Chem. Sens. 2010, 35, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Pérez Ballesta, P.; Baldan, A.; Cancelinha, J. Atmosphere Generation System for the Preparation of Ambient Air Volatile Organic Compound Standard Mixtures. Anal. Chem. 1999, 71, 2241–2245. [Google Scholar] [CrossRef] [PubMed]
- Ricker, N.L.; Muller, C.J.; Craig, I.K. Fuel gas blending benchmark for economic performance evaluation of advanced control and state estimation. J. Process. Control. 2012, 22, 968–974. [Google Scholar] [CrossRef] [Green Version]
- Safdar, M.; Jänis, J.; Sánchez, S. Microfluidic fuel cells for energy generation. Lab Chip 2016, 16, 2754–2758. [Google Scholar] [CrossRef]
- Seong, G.H.; Crooks, R.M. Efficient Mixing and Reactions within Microfluidic Channels Using Microbead-Supported Catalysts. J. Am. Chem. Soc. 2002, 124, 13360–13361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab. Chip 2016, 17, 34–75. [Google Scholar] [CrossRef]
- Christopher, G.F.; Anna, S.L. Microfluidic methods for generating continuous droplet streams. J. Phys. D Appl. Phys. 2007, 40, R319. [Google Scholar] [CrossRef]
- Baroud, C.N.; Willaime, H. Multiphase flows in microfluidics. C. R. Phys. 2004, 5, 547–555. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Middelberg, A.P.J. Two-phase microfluidic flows. Chem. Eng. Sci. 2011, 66, 1394–1411. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chang, C.-L.; Wang, Y.-N.; Fu, L.-M. Microfluidic Mixing: A Review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef] [Green Version]
- Suh, Y.K.; Kang, S. A Review on Mixing in Microfluidics. Micromachines 2010, 1, 82–111. [Google Scholar] [CrossRef] [Green Version]
- Haas-Santo, K.; Pfeifer, P.; Schubert, K.; Zech, T.; Hönicke, D. Experimental evaluation of gas mixing with a static microstructure mixer. Chem. Eng. Sci. 2005, 60, 2955–2962. [Google Scholar] [CrossRef]
- Polinkovsky, M.; Gutierrez, E.; Levchenko, A.; Groisman, A. Fine temporal control of the medium gas content and acidity and on-chip generation of series of oxygen concentrations for cell cultures. Lab. Chip 2009, 9, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Adler, M.; Polinkovsky, M.; Gutierrez, E.; Groisman, A. Generation of oxygen gradients with arbitrary shapes in a microfluidic device. Lab Chip 2010, 10, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.F.; Sinkala, E.; Eddington, D.T. Oxygen gradients for open well cellular cultures via microfluidic substrates. Lab Chip 2010, 10, 2394–2401. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Wan, S.-A.; Hu, Y.-H. Oxygen and nitrogen gases mixing in T-type micromixers visualized and quantitatively characterized using pressure-sensitive paint. Int. J. Heat Mass Transf. 2017, 111, 520–531. [Google Scholar] [CrossRef]
- Tesař, V.R.; Tippetts, J.; Low, Y.-Y. Oscillator Mixer for Chemical Microreactors. In Proceedings of the 9th International Symposium on Flow Visualization, Edinburgh, UK, 22–25 August 2000. [Google Scholar]
- Wilke, C.R.; Lee, C.Y. Estimation of Diffusion Coefficients for Gases and Vapors. Ind. Eng. Chem. 1955, 47, 1253–1257. [Google Scholar] [CrossRef]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef] [Green Version]
- Faanes, A.; Skogestad, S. A systematic approach to the design of buffer tanks. Comput. Chem. Eng. 2000, 24, 1395–1401. [Google Scholar] [CrossRef] [Green Version]
- Tsao, C.-W. Polymer Microfluidics: Simple, Low-Cost Fabrication Process Bridging Academic Lab Research to Commercialized Production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems, 2nd ed.; Cambridge University Press: New York, NY, USA, 1997; ISBN 978-0-521-45078-2. [Google Scholar]
- (PDF) Adsorption of Low-Concentration Formaldehyde from Air by Silver and Copper Nano-Particles Attached on Bamboo-Based Activated Carbon. Available online: https://www.researchgate.net/publication/271305127_Adsorption_of_Low-Concentration_Formaldehyde_from_Air_by_Silver_and_Copper_Nano-Particles_Attached_on_Bamboo-Based_Activated_Carbon (accessed on 8 January 2019).
- Gauf, A.; Navarro, C.; Balch, G.; Hargreaves, L.R.; Khakoo, M.A.; Winstead, C.; McKoy, V. Low-energy elastic electron scattering by acetaldehyde. Phys. Rev. A 2014, 89, 022708. [Google Scholar] [CrossRef] [Green Version]
- Weng, Y.; Qiu, S.; Ma, L.; Liu, Q.; Ding, M.; Zhang, Q.; Zhang, Q.; Wang, T. Jet-Fuel Range Hydrocarbons from Biomass-Derived Sorbitol over Ni-HZSM-5/SBA-15 Catalyst. Catalysts 2015, 5, 2147–2160. [Google Scholar] [CrossRef] [Green Version]









| Short Description/Technology Used | Approach | Applications | Type of Heterogeneity | Total Flow Rate (NmL·min−1) | Mixing Time (s) | Microchip Design | Reference |
|---|---|---|---|---|---|---|---|
| Fluids collision inducing oscillations for mixing liquids or gases | Experimental (liquids) | Fuel technology | Radial | - | - | Fixed | Tesař et al., 2000 [24] |
| Multilamination by using V-shaped microstructures | Experimental | Chemical reaction engineering | Radial | 1000–10,000 | 6 × 10−4 | Modular | Haas-Santo et al., 2005 [25] |
| Microchannels network generating discrete concentrations of O2 in N2 | Experimental | Biotechnology, cell culture | Radial | 16.2 | 4 | Fixed | Polinkovsky et al., 2009 [26] |
| Mixing of 9 gas flows at different O2 concentrations to create O2 concentration gradients | Experimental | Cell culture | Radial | 108 | 24 | Fixed | Adler et al., 2009 [27] |
| Diffusion between parallel flow channels through PDMS layer to create an O2 concentration gradient | Experimental | Cell culture | Radial | 80 | 20 | Modular | Lo et al., 2010 [28] |
| Splitting of the flow between inlet and outlet chambers, followed by a buffer tank | Modelling | Calibration gases generation | Axial | - | 120 | Fixed | Martin et al., 2012 [10] |
| Basic T-shaped mixer | Experimental | Microcombuster, fuel technology | Radial | 5–250 | 3.7 × 10−3 | Modular | Huang et al., 2017 [29] |
| Multistage mixing microchips for pulsed gas flow | Modelling | Gas mixture generation | Axial | 1–100 | 20 | Modular | This work |
| Gas | Molecular Weight (g·mol−1) | Kinetic Diameter σ (Å) | Diffusion Coefficient in Air (cm²·s−1) |
|---|---|---|---|
| Air | 28.97 | 3.71 [8] | 0.178 |
| Formaldehyde (HCHO) | 30.03 | 3.73 [35] | 0.176 |
| Acetaldehyde (CH3CHO) | 44.05 | 7.27 [36] | 0.074 |
| Benzene (C6H6) | 78.11 | 5.85 [37] | 0.089 |
| Toluene (C7H8) | 92.14 | 5.85 [37] | 0.087 |
| Ethylbenzene | 106.17 | 6.00 [37] | 0.083 |
| p-Xylene | 106.16 | 5.85 [37] | 0.086 |
| m-Xylene | 106.16 | 6.80 [37] | 0.071 |
| o-Xylene | 106.16 | 6.80 [37] | 0.071 |
| Naphthalene | 128.17 | 6.20 [37] | 0.078 |
| Flow Rate (NmL·min−1) | 1 | 5 | 10 | 25 | 50 | 100 | Total Volume (mL) |
|---|---|---|---|---|---|---|---|
| Number of Stages | |||||||
| 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 1.686 |
| 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 3.372 |
| 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 5.058 |
| 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6.744 |
| 8 | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | 13.488 |
| 16 | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ | 26.976 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noël, F.; Serra, C.A.; Le Calvé, S. Design of a Novel Axial Gas Pulses Micromixer and Simulations of its Mixing Abilities via Computational Fluid Dynamics. Micromachines 2019, 10, 205. https://doi.org/10.3390/mi10030205
Noël F, Serra CA, Le Calvé S. Design of a Novel Axial Gas Pulses Micromixer and Simulations of its Mixing Abilities via Computational Fluid Dynamics. Micromachines. 2019; 10(3):205. https://doi.org/10.3390/mi10030205
Chicago/Turabian StyleNoël, Florian, Christophe A. Serra, and Stéphane Le Calvé. 2019. "Design of a Novel Axial Gas Pulses Micromixer and Simulations of its Mixing Abilities via Computational Fluid Dynamics" Micromachines 10, no. 3: 205. https://doi.org/10.3390/mi10030205