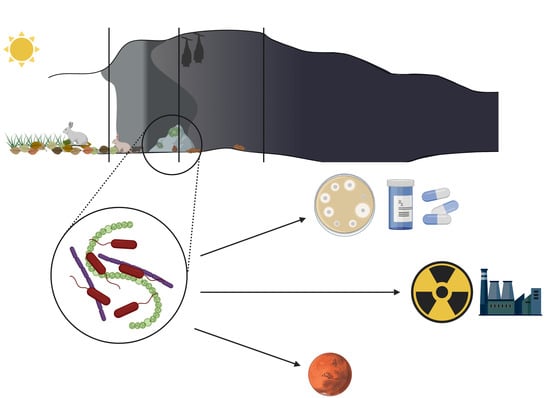
Into the Unknown: Microbial Communities in Caves, Their Role, and Potential Use
Abstract
:1. Introduction
2. Cave as a Specific Habitat
3. Microorganisms in Cave Ecosystems
3.1. Photosynthesis and Lampenflora
3.2. Biomineralization
3.3. Antibacterial and Antifungal Secondary Metabolites
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Yeshurun, R.; Schneller-Pels, N.; Barzilai, O.; Marder, O. Early Upper Paleolithic subsistence in the Levant: Zooarchaeology of the Ahmarian-Aurignacian sequence at Manot Cave, Israel. J. Hum. Evol. 2021, 160, 102619. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, E.; Hunt, C.O.; Reynolds, T.; Abdulmutalb, D.; Asouti, E.; Bennett, P.; Bosch, M.; Burke, A.; Farr, L.; Foley, R.; et al. Issues of theory and method in the analysis of Paleolithic mortuary behavior: A view from Shanidar Cave. Evol. Anthropol. Issues News Rev. 2020, 29, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Jimenez, C. Microbiological and environmental issues in show caves. World J. Microbiol. Biotechnol. 2012, 28, 2453–2464. [Google Scholar] [CrossRef] [PubMed]
- Bontemps, Z.; Alonso, L.; Pommier, T.; Hugoni, M.; Moënne-Loccoz, Y. Microbial ecology of tourist Paleolithic caves. Sci. Total Environ. 2021, 90, 151492. [Google Scholar] [CrossRef]
- Igreja, R.P. Infectious Diseases Associated with Caves. Wilderness Environ. Med. 2011, 22, 115–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Kuisiene, N.; Cheeptham, N. The cave microbiome as a source for drug discovery: Reality or pipe dream? Biochem. Pharmacol. 2017, 134, 18–34. [Google Scholar] [CrossRef]
- Mejía-Ortíz, L.; Christman, M.C.; Pipan, T.; Culver, D.C. What’s the temperature in tropical caves? PLoS ONE 2020, 15, e0237051. [Google Scholar] [CrossRef]
- Riquelme, C.; Dapkevicius, M.d.L.E.; Miller, A.Z.; Charlop-Powers, Z.; Brady, S.; Mason, C.; Cheeptham, N. Biotechnological potential of Actinobacteria from Canadian and Azorean volcanic caves. Appl. Microbiol. Biotechnol. 2017, 101, 843–857. [Google Scholar] [CrossRef] [Green Version]
- Adam, D.; Maciejewska, M.; Naômé, A.; Martinet, L.; Coppieters, W.; Karim, L.; Baurain, D.; Rigali, S. Isolation, Characterization, and Antibacterial Activity of Hard-to-Culture Actinobacteria from Cave Moonmilk Deposits. Antibiotics 2018, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Akob, D.M.; Küsel, K. Where microorganisms meet rocks in the Earth’s Critical Zone. Biogeosciences 2011, 8, 3531–3543. [Google Scholar] [CrossRef] [Green Version]
- Cheeptham, N. Advances and Challenges in Studying Cave Microbial Diversity. In Cave Microbiomes: A Novel Resource for Drug Discovery; Cheeptham, N., Ed.; SpringerBriefs in Microbiology; Springer: New York, NY, USA, 2013; pp. 1–34. ISBN 978-1-4614-5206-5. [Google Scholar]
- Barton, H.A.; Giarrizzo, J.G.; Suarez, P.; Robertson, C.E.; Broering, M.J.; Banks, E.D.; Vaishampayan, P.A.; Venkateswaran, K. Microbial diversity in a Venezuelan orthoquartzite cave is dominated by the Chloroflexi (Class Ktedonobacterales) and Thaumarchaeota Group I.1c. Front. Microbiol. 2014, 5, 615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Cho, Y.-J.; Jung, D.; Jo, K.; Lee, E.-J.; Lee, J.-S. Microbial Diversity in Moonmilk of Baeg-nyong Cave, Korean CZO. Front. Microbiol. 2020, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Gutiérrez, F.; Sánchez-Ortiz, C.A.; Huato-Soberanis, L. Ecological patterns in anchialine caves. PLoS ONE 2018, 13, e0202909. [Google Scholar] [CrossRef] [PubMed]
- Bastian, F.; Jurado, V.; Nováková, A.; Alabouvette, C.; Saiz-Jimenez, C.Y. The microbiology of Lascaux Cave. Microbiology 2020, 156, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Van Hengstum, P.J.; Cresswell, J.N.; Milne, G.A.; Iliffe, T.M. Development of anchialine cave habitats and karst subterranean estuaries since the last ice age. Sci. Rep. 2019, 9, 11907. [Google Scholar] [CrossRef]
- Piano, E.; Bona, F.; Falasco, E.; La Morgia, V.; Badino, G.; Isaia, M. Environmental drivers of phototrophic biofilms in an Alpine show cave (SW-Italian Alps). Sci. Total Environ. 2015, 536, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Borderie, F.; Denis, M.; Barani, A.; Alaoui-Sossé, B.; Aleya, L. Microbial composition and ecological features of phototrophic biofilms proliferating in the Moidons Caves (France): Investigation at the single-cell level. Environ. Sci. Pollut. Res. 2016, 23, 12039–12049. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-Z.; Liu, X.-D.; Jiang, C.-Y.; Liu, S.-J. Cohnella faecalis sp. nov., isolated from animal faeces in a karst cave. Int. J. Syst. Evol. Microbiol. 2019, 69, 572–577. [Google Scholar] [CrossRef]
- Cuezva, S.; Fernandez-Cortes, A.; Porca, E.; Pašić, L.; Jurado, V.; Hernandez-Marine, M.; Serrano-Ortiz, P.; Hermosin, B.; Cañaveras, J.C.; Sanchez-Moral, S.; et al. The biogeochemical role of Actinobacteria in Altamira Cave, Spain. FEMS Microbiol. Ecol. 2012, 81, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Schabereiter-Gurtner, C.; Saiz-Jimenez, C.; Piñar, G.; Lubitz, W.; Rölleke, S. Altamira cave Paleolithic paintings harbor partly unknown bacterial communities. FEMS Microbiol. Lett. 2002, 211, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monro, A.K.; Bystriakova, N.; Fu, L.; Wen, F.; Wei, Y. Discovery of a diverse cave flora in China. PLoS ONE 2018, 13, e0190801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussotti, S.; Di Franco, A.; Bianchi, C.N.; Chevaldonné, P.; Egea, L.; Fanelli, E.; Lejeusne, C.; Musco, L.; Navarro-Barranco, C.; Pey, A.; et al. Fish mitigate trophic depletion in marine cave ecosystems. Sci. Rep. 2018, 8, 9193. [Google Scholar] [CrossRef]
- Gross, J.B.; Sun, D.A.; Carlson, B.M.; Brodo-Abo, S.; Protas, M.E. Developmental Transcriptomic Analysis of the Cave-Dwelling Crustacean, Asellus aquaticus. Genes 2020, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Riquelme, C.; Hathaway, J.J.M.; Dapkevicius, M.d.L.N.E.; Miller, A.Z.; Kooser, A.; Northup, D.E.; Jurado, V.; Fernandez, O.; Saiz-Jimenez, C.; Cheeptham, N. Actinobacterial Diversity in Volcanic Caves and Associated Geomicrobiological Interactions. Front. Microbiol. 2015, 6, 1342. [Google Scholar] [CrossRef] [Green Version]
- Jaroszewicz, W.; Bielańska, P.; Lubomska, D.; Kosznik-Kwaśnicka, K.; Golec, P.; Grabowski, Ł.; Wieczerzak, E.; Dróżdż, W.; Gaffke, L.; Pierzynowska, K.; et al. Antibacterial, Antifungal and Anticancer Activities of Compounds Produced by Newly Isolated Streptomyces Strains from the Szczelina Chochołowska Cave (Tatra Mountains, Poland). Antibiotics 2021, 10, 1212. [Google Scholar] [CrossRef]
- Addesso, R.; Gonzalez-Pimentel, J.L.; D’Angeli, I.M.; De Waele, J.; Saiz-Jimenez, C.; Jurado, V.; Miller, A.Z.; Cubero, B.; Vigliotta, G.; Baldantoni, D. Microbial Community Characterizing Vermiculations from Karst Caves and Its Role in Their Formation. Microb. Ecol. 2021, 81, 884–896. [Google Scholar] [CrossRef]
- Moldovan, O.T.; Kováč, Ľ.; Halse, S. Cave Ecology; Ecological Studies 235; Moldovan, O.T., Kováč, Ľ., Halse, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-98850-4. [Google Scholar]
- De Paula, C.C.P.; Bichuette, M.E.; Seleghim, M.H.R. Nutrient availability in tropical caves influences the dynamics of microbial biomass. MicrobiologyOpen 2020, 9, e1044. [Google Scholar] [CrossRef]
- Roldán, M.; Hernández-Mariné, M. Exploring the secrets of the three-dimensional architecture of phototrophic biofilms in caves. Int. J. Speleol. 2009, 38, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Cyske, Z.; Jaroszewicz, W.; Żabińska, M.; Lorenc, P.; Sochocka, M.; Bielańska, P.; Grabowski, Ł.; Gaffke, L.; Pierzynowska, K.; Węgrzyn, G. Unexplored potential: Biologically active compounds produced by microorganisms from hard-to-reach environments and their applications. Acta Biochim. Pol. 2020, 68, 565–574. [Google Scholar] [CrossRef]
- Cennamo, P.; Marzano, C.; Ciniglia, C.; Pinto, G.; Cappelletti, P.; Caputo, P.; Pollio, A. A survey of the algal flora of anthropogenic caves of Campi Flegrei (Naples, Italy) archeological district. J. Cave Karst Stud. 2012, 174, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Popović, S.; Krizmanić, J.; Vidaković, D.; Karadžić, V.; Milovanović, Ž.; Pećić, M.; Simić, G.S. Biofilms in caves: Easy method for the assessment of dominant phototrophic groups/taxa in situ. Environ. Monit. Assess. 2020, 192, 720. [Google Scholar] [CrossRef] [PubMed]
- Popović, S.; Nikolić, N.; Jovanović, J.; Predojević, D.; Trbojević, I.; Manić, L.; Subakov-Simić, G. Cyanobacterial and algal abundance and biomass in cave biofilms and relation to environmental and biofilm parameters. Int. J. Speleol. 2019, 48, 49–61. [Google Scholar] [CrossRef]
- Pfendler, S.; Munch, T.; Bousta, F.; Alaoui-Sosse, L.; Aleya, L.; Alaoui-Sossé, B. Bleaching of biofilm-forming algae induced by UV-C treatment: A preliminary study on chlorophyll degradation and its optimization for an application on cultural heritage. Environ. Sci. Pollut. Res. 2018, 25, 14097–14105. [Google Scholar] [CrossRef] [PubMed]
- Czerwik-Marcinkowska, J.; Wojciechowska, A.; Massalski, A. Biodiversity of Limestone Caves: Aggregations of Aerophytic Algae and Cyanobacteria in Relation to Site Factors. Pol. J. Ecol. 2015, 63, 481–499. [Google Scholar] [CrossRef]
- Macedo, M.F.; Miller, A.Z.; Dionísio, A.; Saiz-Jimenez, C. Biodiversity of cyanobacteria and green algae on monuments in the Mediterranean Basin: An overview. Microbiology 2009, 155, 3476–3490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borderie, F.; Alaoui-Sossé, B.; Aleya, L. Heritage materials and biofouling mitigation through UV-C irradiation in show caves: State-of-the-art practices and future challenges. Environ. Sci. Pollut. Res. 2015, 22, 4144–4172. [Google Scholar] [CrossRef] [PubMed]
- Mulec, J.; Kosi, G.; Danijel, A.; Ek, V. Characterization of cave aerophytic algal communities and effects of irradiance levels on production of pigments. J. Cave Karst Stud. 2008, 70, 3–12. [Google Scholar]
- Popović, S.; Simic, G.S.; Stupar, M.; Unković, N.; Predojević, D.; Jovanović, J.; Grbić, M. Cyanobacteria, algae and microfungi present in biofilm from Božana Cave (Serbia). Int. J. Speleol. 2015, 44, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Albertano, P. Cyanobacterial Biofilms in Monuments and Caves. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 317–343. ISBN 978-94-007-3855-3. [Google Scholar]
- Cirés, S.; Casero, M.C.; Quesada, A. Toxicity at the Edge of Life: A Review on Cyanobacterial Toxins from Extreme Environments. Mar. Drugs 2017, 15, 233. [Google Scholar] [CrossRef]
- Heidari, F.; Riahi, H.; Yousefzadi, M.; Asadi, M. Antimicrobial activity of cyanobacteria isolated from hot spring of Geno. Middle East J. Sci. Res. 2012, 12, 336–339. [Google Scholar] [CrossRef]
- Felczykowska, A.; Pawlik, A.; Mazur-Marzec, H.; Toruńska-Sitarz, A.; Narajczyk, M.; Richert, M.; Węgrzyn, G.; Herman-Antosiewicz, A. Selective inhibition of cancer cells’ proliferation by compounds included in extracts from Baltic Sea cyanobacteria. Toxicon 2015, 108, 1–10. [Google Scholar] [CrossRef]
- Lamprinou, V.; Tryfinopoulou, K.; Velonakis, E.; Vatopoulos, A.; Antonopoulou, S.; Fragopoulou, E.; Pantazidou, A.; Economou-Amilli, A. Cave Cyanobacteria showing antibacterial activity. Int. J. Speleol. 2015, 44, 1. [Google Scholar] [CrossRef] [Green Version]
- Felczykowska, A.; Bloch, S.K.; Nejman-Faleńczyk, B.; Barańska, S. Metagenomic approach in the investigation of new bioactive compounds in the marine environment. Acta Biochim. Pol. 2012, 59, 501–505. [Google Scholar] [CrossRef] [Green Version]
- Baqué, M.; de Vera, J.-P.; Rettberg, P.; Billi, D. The BOSS and BIOMEX space experiments on the EXPOSE-R2 mission: Endurance of the desert cyanobacterium Chroococcidiopsis under simulated space vacuum, Martian atmosphere, UVC radiation and temperature extremes. Acta Astronaut. 2013, 91, 180–186. [Google Scholar] [CrossRef]
- Billi, D.; Baqué, M.; Smith, H.D.; McKay, C.P. Cyanobacteria from Extreme Deserts to Space. Adv. Microbiol. 2013, 3, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Mulec, J.; Kosi, G. Lampenflora algae and methods of growth control. J. Cave Karst Stud. 2009, 71, 109–115. [Google Scholar]
- De Luca, D.; Caputo, P.; Perfetto, T.; Cennamo, P. Characterisation of Environmental Biofilms Colonising Wall Paintings of the Fornelle Cave in the Archaeological Site of Cales. Int. J. Environ. Res. Public Health 2021, 18, 8048. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Lithophytic algae: A major threat to the karst formation of show caves. J. Appl. Phycol. 2000, 12, 309–315. [Google Scholar] [CrossRef]
- Trinh, D.A.; Trinh, Q.H.; Tran, N.; Guinea, J.G.; Mattey, D. Eco-friendly Remediation of Lampenflora on Speleothems in Tropical Karst Caves. J. Cave Karst Stud. 2018, 80, 1–12. [Google Scholar] [CrossRef]
- Cennamo, P.; Pasquino, N.; Ciniglia, C.; Moretti, A.; Caputo, P. Use of radiofrequency electromagnetic radiation to remove biofilms from canvasses. Aerobiologia 2020, 36, 541–549. [Google Scholar] [CrossRef]
- Pfendler, S.; Einhorn, O.; Karimi, B.; Bousta, F.; Cailhol, D.; Alaoui-Sosse, L.; Alaoui-Sosse, B.; Aleya, L. UV-C as an efficient means to combat biofilm formation in show caves: Evidence from the La Glacière Cave (France) and laboratory experiments. Environ. Sci. Pollut. Res. 2017, 24, 24611–24623. [Google Scholar] [CrossRef]
- Borderie, F.; Tête, N.; Cailhol, D.; Alaoui-Sehmer, L.; Bousta, F.; Rieffel, D.; Aleya, L.; Alaoui-Sossé, B. Factors driving epilithic algal colonization in show caves and new insights into combating biofilm development with UV-C treatments. Sci. Total Environ. 2014, 484, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Mulec, J. Human impact on underground cultural and natural heritage sites, biological parameters of monitoring and remediation actions for insensitive surfaces: Case of Slovenian show caves. J. Nat. Conserv. 2014, 22, 132–141. [Google Scholar] [CrossRef]
- Cennamo, P.; Caputo, P.; Giorgio, A.; Moretti, A.; Pasquino, N. Biofilms on Tuff Stones at Historical Sites: Identification and Removal by Nonthermal Effects of Radiofrequencies. Microb. Ecol. 2013, 66, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J.; Gerlach, R.; Lauchnor, E.; Mitchell, A.C.; Cunningham, A.B.; Spangler, L. Engineered applications of ureolytic biomineralization: A review. Biofouling 2013, 29, 715–733. [Google Scholar] [CrossRef] [Green Version]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Hesse, A.; Heimbach, D. Causes of phosphate stone formation and the importance of metaphylaxis by urinary acidification: A review. World J. Urol. 1999, 17, 308–315. [Google Scholar] [CrossRef]
- Anbu, P.; Kang, C.-H.; Shin, Y.-J.; So, J.-S. Formations of calcium carbonate minerals by bacteria and its multiple applications. SpringerPlus 2016, 5, 250. [Google Scholar] [CrossRef] [Green Version]
- Crichton, R. Chapter 19—Biomineralization. In Biological Inorganic Chemistry, 3rd ed.; Crichton, R., Ed.; Academic Press: London, UK, 2019; pp. 517–544. ISBN 978-0-12-811741-5. [Google Scholar]
- Carmichael, M.J.; Carmichael, S.K.; Santelli, C.M.; Strom, A.; Bräuer, S.L. Mn(II)-oxidizing Bacteria are Abundant and Environmentally Relevant Members of Ferromanganese Deposits in Caves of the Upper Tennessee River Basin. Geomicrobiol. J. 2013, 30, 779–800. [Google Scholar] [CrossRef]
- Joester, D.; Brooker, L.R. The Chiton Radula: A Model System for Versatile Use of Iron Oxides. In Iron Oxides; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 177–206. ISBN 978-3-527-69139-5. [Google Scholar]
- Le Moigne, F.A.C. Pathways of Organic Carbon Downward Transport by the Oceanic Biological Carbon Pump. Front. Mar. Sci. 2019, 6, 634. [Google Scholar] [CrossRef] [Green Version]
- Bosak, T. Calcite Precipitation, Microbially Induced. In Encyclopedia of Geobiology; Reitner, J., Thiel, V., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 223–227. ISBN 978-1-4020-9212-1. [Google Scholar]
- Stabnikov, V.; Jian, C.; Ivanov, V.; Li, Y. Halotolerant, alkaliphilic urease-producing bacteria from different climate zones and their application for biocementation of sand. World J. Microbiol. Biotechnol. 2013, 29, 1453–1460. [Google Scholar] [CrossRef]
- Enyedi, N.T.; Makk, J.; Kótai, L.; Berényi, B.; Klébert, S.; Sebestyén, Z.; Molnár, Z.; Borsodi, A.K.; Leél-Őssy, S.; Demény, A.; et al. Cave bacteria-induced amorphous calcium carbonate formation. Sci. Rep. 2020, 10, 8696. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Bindschedler, S.; Daniels, A.; Verrecchia, E.; Cailleau, G. Microbiological activities in moonmilk monitored using isothermal microcalorimetry (Cave of Vers chez le Brandt, Neuchatel, Switzerland). J. Cave Karst Stud. 2012, 74, 116–126. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Falaleeva, M.I.; Klimova, M.A.; Parfenova, N.V. Isolation and properties of malate dehydrogenase from Meso- and thermophilic bacteria. Appl. Biochem. Microbiol. 2006, 42, 241–245. [Google Scholar] [CrossRef]
- Maciejewska, M.; Adam, D.; Naômé, A.; Martinet, L.; Tenconi, E.; Całusińska, M.; Delfosse, P.; Hanikenne, M.; Baurain, D.; Compère, P.; et al. Assessment of the Potential Role of Streptomyces in Cave Moonmilk Formation. Front. Microbiol. 2017, 8, 1181. [Google Scholar] [CrossRef] [Green Version]
- Bullen, H.A.; Oehrle, S.A.; Bennett, A.F.; Taylor, N.M.; Barton, H.A. Use of Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy to Identify Microbial Metabolic Products on Carbonate Mineral Surfaces. Appl. Environ. Microbiol. 2008, 74, 4553–4559. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.S. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef] [Green Version]
- Addesso, R.; Bellino, A.; D’Angeli, I.M.; De Waele, J.; Miller, A.Z.; Carbone, C.; Baldantoni, D. Vermiculations from karst caves: The case of Pertosa-Auletta system (Italy). Catena 2019, 182, 104178. [Google Scholar] [CrossRef]
- Jones, D.S.; Lyon, E.H.; Macalady, J.L. Geomicrobiology of Biovermiculations from the Frasassi Cave System, Italy. J. Cave Karst Stud. 2008, 70, 78–93. [Google Scholar]
- Bini, A.; Gori, M.C.; Gori, S. A critical review of hypotheses on the origin of vermiculations. Int. J. Speleol. 1978, 10, 11–33. [Google Scholar] [CrossRef] [Green Version]
- Spilde, M.N.; Northup, D.E.; Boston, P.J.; Schelble, R.T.; Dano, K.E.; Crossey, L.J.; Dahm, C.N. Geomicrobiology of Cave Ferromanganese Deposits: A Field and Laboratory Investigation. Geomicrobiol. J. 2005, 22, 99–116. [Google Scholar] [CrossRef]
- Stabnikov, V.; Naeimi, M.; Ivanov, V.; Chu, J. Formation of water-impermeable crust on sand surface using biocement. Cem. Concr. Res. 2011, 41, 1143–1149. [Google Scholar] [CrossRef]
- Kang, C.-H.; Kwon, Y.-J.; So, J.-S. Soil Bioconsolidation through Microbially Induced Calcite Precipitation by Lysinibacillus sphaericus WJ-8. Geomicrobiol. J. 2016, 33, 473–478. [Google Scholar] [CrossRef]
- Achal, V.; Kawasaki, S. Biogrout: A Novel Binding Material for Soil Improvement and Concrete Repair. Front. Microbiol. 2016, 7, 314. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Zabihi, M.; Tahmasbi, M.; Bastami, T.R. Effect of adsorbents and chemical treatments on the removal of strontium from aqueous solutions. J. Hazard. Mater. 2010, 182, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Taylor, J.L.; Wendt, L.M.; Reed, D.W.; Smith, R.W. Evaluating the Potential of Native Ureolytic Microbes to Remediate a 90Sr Contaminated Environment. Environ. Sci. Technol. 2010, 44, 7652–7658. [Google Scholar] [CrossRef] [PubMed]
- Achal, V.; Pan, X.; Zhang, D. Bioremediation of strontium (Sr) contaminated aquifer quartz sand based on carbonate precipitation induced by Sr resistant Halomonas sp. Chemosphere 2012, 89, 764–768. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Lee, D.-J.; Kumari, D.; Zhang, D. Remediation of Cr(VI) from chromium slag by biocementation. Chemosphere 2013, 93, 1352–1358. [Google Scholar] [CrossRef]
- Li, M.; Cheng, X.; Guo, H. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85. [Google Scholar] [CrossRef]
- Fang, B.-Z.; Salam, N.; Han, M.-X.; Jiao, J.-Y.; Cheng, J.; Wei, D.-Q.; Xiao, M.; Li, W.-J. Insights on the Effects of Heat Pretreatment, pH, and Calcium Salts on Isolation of Rare Actinobacteria from Karstic Caves. Front. Microbiol. 2017, 8, 1535. [Google Scholar] [CrossRef] [Green Version]
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis. Mon. 2020, 66, 100971. [Google Scholar] [CrossRef] [PubMed]
- Gunjal, V.B.; Thakare, R.; Chopra, S.; Reddy, D.S. Teixobactin: A Paving Stone toward a New Class of Antibiotics? J. Med. Chem. 2020, 63, 12171–12195. [Google Scholar] [CrossRef] [PubMed]
- Górski, A.; Borysowski, J.; Międzybrodzki, R. Phage Therapy: Towards a Successful Clinical Trial. Antibiotics 2020, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Zada, S.; Sajjad, W.; Rafiq, M.; Ali, S.; Hu, Z.; Wang, H.; Cai, R. Cave Microbes as a Potential Source of Drugs Development in the Modern Era. Microb. Ecol. 2021, 1–14. [Google Scholar] [CrossRef]
- Maciejewska, M.; Adam, D.; Martinet, L.; Naômé, A.; Całusińska, M.; Delfosse, P.; Carnol, M.; Barton, H.A.; Hayette, M.-P.; Smargiasso, N.; et al. A Phenotypic and Genotypic Analysis of the Antimicrobial Potential of Cultivable Streptomyces Isolated from Cave Moonmilk Deposits. Front. Microbiol. 2016, 7, 1455. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, S.; Chattopadhyay, M.; Grossart, H.-P. The multifaceted roles of antibiotics and antibiotic resistance in nature. Front. Microbiol. 2013, 4, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paun, V.I.; Lavin, P.; Chifiriuc, M.C.; Purcarea, C. First report on antibiotic resistance and antimicrobial activity of bacterial isolates from 13,000-year old cave ice core. Sci. Rep. 2021, 11, 514. [Google Scholar] [CrossRef]
- Selim, M.S.M.; Abdelhamid, S.A.; Mohamed, S.S. Secondary metabolites and biodiversity of actinomycetes. J. Genet. Eng. Biotechnol. 2021, 19, 72. [Google Scholar] [CrossRef]
- Jakubiec-Krzesniak, K.; Rajnisz-Mateusiak, A.; Guspiel, A.; Ziemska, J.; Solecka, J. Secondary Metabolites of Actinomycetes and their Antibacterial, Antifungal and Antiviral Properties. Pol. J. Microbiol. 2018, 67, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Quinn, G.A.; Banat, A.M.; Abdelhameed, A.M.; Banat, I.M.Y. 2020 Streptomyces from traditional medicine: Sources of new innovations in antibiotic discovery. J. Med. Microbiol. 2020, 69, 1040–1048. [Google Scholar] [CrossRef]
- Stankovic, N.; Radulovic, V.; Petkovic, M.; Vuckovic, I.; Jadranin, M.; Vasiljevic, B.; Nikodinovic-Runic, J. Streptomyces sp. JS520 produces exceptionally high quantities of undecylprodigiosin with antibacterial, antioxidative, and UV-protective properties. Appl. Microbiol. Biotechnol. 2012, 96, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Axenov-Gibanov, D.V.; Voytsekhovskaya, I.V.; Tokovenko, B.T.; Protasov, E.S.; Gamaiunov, S.V.; Rebets, Y.V.; Luzhetskyy, A.N.; Timofeyev, M.A. Actinobacteria Isolated from an Underground Lake and Moonmilk Speleothem from the Biggest Conglomeratic Karstic Cave in Siberia as Sources of Novel Biologically Active Compounds. PLoS ONE 2016, 11, e0149216. [Google Scholar] [CrossRef]
- Voytsekhovskaya, I.V.; Axenov-Gribanov, D.V.; Murzina, S.A.; Pekkoeva, S.N.; Protasov, E.S.; Gamaiunov, S.V.; Timofeyev, M.A. Estimation of antimicrobial activities and fatty acid composition of actinobacteria isolated from water surface of underground lakes from Badzheyskaya and Okhotnichya caves in Siberia. PeerJ 2018, 6, e5832. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Guo, L.; Chen, C.; Liu, S.; Zhang, L.; Dai, S.; He, Q.; You, X.; Hu, X.; Tuo, L.; et al. Xiakemycin A, a novel pyranonaphthoquinone antibiotic, produced by the Streptomyces sp. CC8-201 from the soil of a karst cave. J. Antibiot. 2015, 68, 771–774. [Google Scholar] [CrossRef] [Green Version]
- Rajput, Y.; Biswas, J.; Rai, V. Potentiality Test in Antimicrobial Activity and Antibiotic Sensitivity of Subterranean Streptomyces Strains Isolated from Kotumsar Cave of India. Int. J. Biol. Chem. 2012, 6, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Yücel, S.; Yamac, M. Selection of Streptomyces isolates from Turkish karstic caves against antibiotic resistant microorganisms. Pak. J. Pharm. Sci. 2010, 23, 1–6. [Google Scholar]
- Herold, K.; Gollmick, F.A.; Groth, I.; Roth, M.; Menzel, K.-D.; Möllmann, U.; Gräfe, U.; Hertweck, C. Cervimycin A–D: A Polyketide Glycoside Complex from a Cave Bacterium Can Defeat Vancomycin Resistance. Chem.—A Eur. J. 2005, 11, 5523–5530. [Google Scholar] [CrossRef]
- Lee, S.D.; Kim, E.S.; Roe, J.H.; Kim, J.; Kang, S.O.; Hah, Y.C. Saccharothrix violacea sp. nov., isolated from a gold mine cave, and Saccharothrix albidocapillata comb. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 1315–1323. [Google Scholar] [CrossRef] [Green Version]
- Ningthoujam, D.; Sanasam, S.; Salam, N. Screening of Actinomycete Isolates from Niche Habitats in Manipur for Antibiotic Activity. Am. J. Biochem. Biotechnol. 2009, 5, 221–225. [Google Scholar] [CrossRef]
- Nakaew, N.; Pathom-aree, W.; Lumyong, S. First Record of the Isolation, Identification and Biological Activity of a New Strain of Spirillospora albida from Thai Cave Soil. Actinomycetologica 2009, 23, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tomova, I.; Lazarkevich, I.; Tomova, A.; Kambourova, M.; Vasileva-Tonkova, E. Diversity and biosynthetic potential of culturable aerobic heterotrophic bacteria isolated from Magura Cave, Bulgaria. Int. J. Speleol. 2013, 42, 65–76. [Google Scholar] [CrossRef]
- Derewacz, D.K.; McNees, C.R.; Scalmani, G.; Covington, C.L.; Shanmugam, G.; Marnett, L.J.; Polavarapu, P.L.; Bachmann, B.O. Structure and Stereochemical Determination of Hypogeamicins from a Cave-Derived Actinomycete. J. Nat. Prod. 2014, 77, 1759–1763. [Google Scholar] [CrossRef] [Green Version]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.Y.; Yoon, K.; Lee, J.I.; Mitchell, R.J. Violacein: Properties and Production of a Versatile Bacterial Pigment. BioMed. Res. Int. 2015, 2015, 465056. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.Y.; Im, H.; Mitchell, R.J. Violacein and bacterial predation: Promising alternatives for priority multidrug resistant human pathogens. Future Microbiol. 2017, 12, 835–838. [Google Scholar] [CrossRef]
- Ghosh, S.; Kam, G.; Nijjer, M.; Stenner, C.; Cheeptham, N. Culture dependent analysis of bacterial diversity in Canada’s Raspberry Rising Cave revealed antimicrobial properties. Int. J. Speleol. 2020, 49, 43–53. [Google Scholar] [CrossRef]
- Friedrich, I.; Hollensteiner, J.; Schneider, D.; Poehlein, A.; Hertel, R.; Daniel, R. First Complete Genome Sequences of Janthinobacterium lividum EIF1 and EIF2 and Their Comparative Genome Analysis. Genome Biol. Evol. 2020, 12, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Paun, V.I.; Icaza, G.; Lavin, P.; Marin, C.; Tudorache, A.; Perşoiu, A.; Dorador, C.; Purcarea, C. Total and Potentially Active Bacterial Communities Entrapped in a Late Glacial Through Holocene Ice Core From Scarisoara Ice Cave, Romania. Front. Microbiol. 2019, 10, 1193. [Google Scholar] [CrossRef]
- Gálvez, A.; Maqueda, M.; Martínez-Bueno, M.; Lebbadi, M.; Valdivia, E. Isolation and physico-chemical characterization of an antifungal and antibacterial peptide produced by Bacillus licheniformis A12. Appl. Microbiol. Biotechnol. 1993, 39, 438–442. [Google Scholar] [CrossRef]
- Salwan, R.; Sharma, V. Molecular and biotechnological aspects of secondary metabolites in actinobacteria. Microbiol. Res. 2020, 231, 126374. [Google Scholar] [CrossRef]
- Bibb, M.J. Regulation of secondary metabolism in streptomycetes. Curr. Opin. Microbiol. 2005, 8, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Hoskisson, P.A.; Fernández-Martínez, L.T. Regulation of specialised metabolites in Actinobacteria—Expanding the paradigms. Environ. Microbiol. Rep. 2018, 10, 231–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klusaite, A.; Vickackaite, V.; Vaitkeviciene, B.; Karnickaite, R.; Bukelskis, D.; Kieraite-Aleksandrova, I.; Kuisiene, N. Characterization of antimicrobial activity of culturable bacteria isolated from Krubera-Voronja Cave. Int. J. Speleol. 2016, 45, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Glass, T.W.; Breed, G.A.; Iwahana, G.; Kynoch, M.C.; Robards, M.D.; Williams, C.T.; Kielland, K. Permafrost ice caves: An unrecognized microhabitat for Arctic wildlife. Ecology 2021, 102, e03276. [Google Scholar] [CrossRef]
- Lavoie, K.H.; Winter, A.S.; Read, K.J.H.; Hughes, E.M.; Spilde, M.N.; Northup, D.E. Comparison of bacterial communities from lava cave microbial mats to overlying surface soils from Lava Beds National Monument, USA. PLoS ONE 2017, 12, e0169339. [Google Scholar] [CrossRef]
- Riquelme, C.; Rigal, F.; Hathaway, J.J.M.; Northup, D.E.; Spilde, M.N.; Borges, P.A.V.; Gabriel, R.; Amorim, I.R.; Dapkevicius, M.d.L.N.E. Cave microbial community composition in oceanic islands: Disentangling the effect of different colored mats in diversity patterns of Azorean lava caves. FEMS Microbiol. Ecol. 2015, 91, 12. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosznik-Kwaśnicka, K.; Golec, P.; Jaroszewicz, W.; Lubomska, D.; Piechowicz, L. Into the Unknown: Microbial Communities in Caves, Their Role, and Potential Use. Microorganisms 2022, 10, 222. https://doi.org/10.3390/microorganisms10020222
Kosznik-Kwaśnicka K, Golec P, Jaroszewicz W, Lubomska D, Piechowicz L. Into the Unknown: Microbial Communities in Caves, Their Role, and Potential Use. Microorganisms. 2022; 10(2):222. https://doi.org/10.3390/microorganisms10020222
Chicago/Turabian StyleKosznik-Kwaśnicka, Katarzyna, Piotr Golec, Weronika Jaroszewicz, Daria Lubomska, and Lidia Piechowicz. 2022. "Into the Unknown: Microbial Communities in Caves, Their Role, and Potential Use" Microorganisms 10, no. 2: 222. https://doi.org/10.3390/microorganisms10020222