The Binding of Phosphorus Species at Goethite: A Joint Experimental and Theoretical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Goethite Preparation and Adsorption Experiments
2.2. Molecular Modeling and Computational Details
3. Results and Discussion
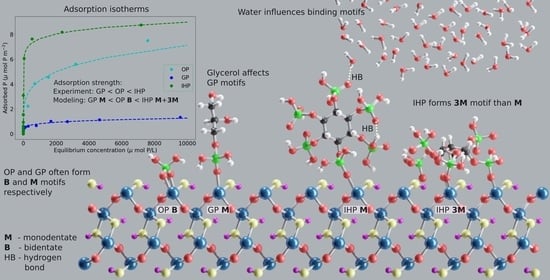
3.1. Adsorption Isotherms
3.2. Molecular Modeling of P Interactions at the Goethite–Water Interface
3.2.1. Orthophosphate
3.2.2. Glycerolphosphate
3.2.3. Inositolhexaphosphate
3.3. Discussion of Experimental and Modeling Results
4. Summarizing Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tisdale, S.L. (Ed.) Soil Fertility and Fertilizers, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1993. [Google Scholar]
- Andersen, D.S.; Helmers, M.J.; Burns, R.T. Phosphorus Sorption Capacity of Six Iowa Soils before and after Five Years of Use as Vegetative Treatment Areas. Appl. Eng. Agric. 2015, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, T.; O’Halloran, I.; Tan, C.; Hu, Q. A Phosphorus Sorption Index and Its Use to Estimate Leaching of Dissolved Phosphorus from Agricultural Soils in Ontario. Geoderma 2016, 274, 79–87. [Google Scholar] [CrossRef]
- Roy, E.D.; Willig, E.; Richards, P.D.; Martinelli, L.A.; Vazquez, F.F.; Pegorini, L.; Spera, S.A.; Porder, S. Soil Phosphorus Sorption Capacity after Three Decades of Intensive Fertilization in Mato Grosso, Brazil. Agric. Ecosyst. Environ. 2017, 249, 206–214. [Google Scholar] [CrossRef]
- Everett, D.H. Manual of Symbols and Terminology for Physicochemical Quantities and Units, Appendix II: Definitions, Terminology and Symbols in Colloid and Surface Chemistry. Pure Appl. Chem. 1972, 31, 577–638. [Google Scholar] [CrossRef]
- Urrutia, O.; Guardado, I.; Erro, J.; Mandado, M.; García-Mina, J.M. Theoretical Chemical Characterization of Phosphate-Metal–Humic Complexes and Relationships with Their Effects on Both Phosphorus Soil Fixation and Phosphorus Availability for Plants. J. Sci. Food Agric. 2013, 93, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Gros, P.; Ahmed, A.A.; Kühn, O.; Leinweber, P. Influence of Metal Ions on Glyphosate Detection by FMOC-Cl. Environ. Model. Assess. 2019, 191, 244. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.; Abraham, M.; Amelung, W.; Baum, C.; Bol, R.; Kühn, O.; Lewandowski, H.; Niederberger, J.; Oelmann, Y.; Rüger, C.; et al. Innovative Methods in Soil Phosphorus Research: A Review. J. Soil Sci. Plant Nutr. 2015, 178, 43–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.A.; Leinweber, P.; Kühn, O. Unravelling the Nature of Glyphosate Binding to Goethite Surfaces by Ab Initio Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2018, 20, 1531–1539. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.A.; Gypser, S.; Leinweber, P.; Freese, D.; Kühn, O. Infrared Spectroscopic Characterization of Phosphate Binding at the Goethite-Water Interface. Phys. Chem. Chem. Phys. 2019, 21, 4421–4434. [Google Scholar] [CrossRef] [Green Version]
- Ganta, P.B.; Kühn, O.; Ahmed, A.A. QM/MM Simulations of Organic Phosphorus Adsorption at the Diaspore–Water Interface. Phys. Chem. Chem. Phys. 2019, 21, 24316–24325. [Google Scholar] [CrossRef]
- Gros, P.; Ahmed, A.; Kühn, O.; Leinweber, P. Glyphosate Binding in Soil as Revealed by Sorption Experiments and Quantum-Chemical Modeling. Sci. Total Environ. 2017, 586, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Gros, P.; Kühn, O.; Leinweber, P. Molecular Level Investigation of the Role of Peptide Interactions in the Glyphosate Analytics. Chemosphere 2018, 196, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Arai, Y.; Sparks, D. Phosphate Reaction Dynamics in Soils and Soil Components: A Multiscale Approach. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2007; Volume 94, pp. 135–179. [Google Scholar] [CrossRef]
- Arai, Y.; Sparks, D. ATR–FTIR Spectroscopic Investigation on Phosphate Adsorption Mechanisms at the Ferrihydrite–Water Interface. J. Colloid Interface Sci. 2001, 241, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Barrow, N.J. A Mechanistic Model for Describing the Sorption and Desorption of Phosphate by Soil. J. Soil Sci. 1983, 34, 733–750. [Google Scholar] [CrossRef]
- Chitrakar, R.; Tezuka, S.; Sonoda, A.; Sakane, K.; Ooi, K.; Hirotsu, T. Phosphate Adsorption on Synthetic Goethite and Akaganeite. J. Colloid Interface Sci. 2006, 298, 602–608. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of Soil Inorganic P in the Rhizosphere as Affected by Root-Induced Chemical Changes: A Review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Ryan, J.; Curtin, D.; Cheema, M. Significance of Iron Oxides and Calcium Carbonate Particle Size in Phosphate Sorption by Calcareous Soils. Soil Sci. Soc. Am. J. 1985, 49, 74–76. [Google Scholar] [CrossRef]
- Baken, S.; Verbeeck, M.; Verheyen, D.; Diels, J.; Smolders, E. Phosphorus Losses from Agricultural Land to Natural Waters Are Reduced by Immobilization in Iron-Rich Sediments of Drainage Ditches. Water Res. 2015, 71, 160–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortoluzzi, E.C.; Pérez, C.A.; Ardisson, J.D.; Tiecher, T.; Caner, L. Occurrence of Iron and Aluminum Sesquioxides and Their Implications for the P Sorption in Subtropical Soils. Appl. Clay Sci. 2015, 104, 196–204. [Google Scholar] [CrossRef]
- Torrent, J.; Schwertmann, U.; Barron, V. Fast and Slow Phosphate Sorption by Goethite-Rich Natural Materials. Clays Clay Miner. 1992, 40, 14–21. [Google Scholar] [CrossRef]
- Luengo, C.; Brigante, M.; Antelo, J.; Avena, M. Kinetics of Phosphate Adsorption on Goethite: Comparing Batch Adsorption and ATR-IR Measurements. J. Colloid Interface Sci. 2006, 300, 511–518. [Google Scholar] [CrossRef]
- McLaughlin, J.; Ryden, J.; Syers, J. Development and Evaluation of a Kinetic Model to Describe Phosphate Sorption by Hydrous Ferric Oxide Gel. Geoderma 1977, 18, 295–307. [Google Scholar] [CrossRef]
- Willett, I.R.; Chartres, C.J.; Nguyen, T.T. Migration of Phosphate into Aggregated Particles of Ferrihydrite. J. Soil Sci. 1988, 39, 275–282. [Google Scholar] [CrossRef]
- Torrent, J. Interactions between Phosphate and Iron Oxide. In Soils and Environment; Catena Verlag: Reiskirchen, Germany, 1997; Volume 30, pp. 321–344. [Google Scholar]
- Strauss, R.; Brummer, G.; Barrow, N. Effects of Crystallinity of Goethite: II. Rates of Sorption and Desorption of Phosphate. Eur. J. Soil Sci. 1997, 48, 101–114. [Google Scholar] [CrossRef]
- Neupane, G.; Donahoe, R.J.; Arai, Y. Kinetics of Competitive Adsorption/Desorption of Arsenate and Phosphate at the Ferrihydrite– Water Interface. Chem. Geol. 2014, 368, 31–38. [Google Scholar] [CrossRef]
- Parfitt, R.L. Phosphate Reactions with Natural Allophane, Ferrihydrite and Goethite. J. Soil Sci. 1989, 40, 359–369. [Google Scholar] [CrossRef]
- Langmuir, D. Aqueous Environmental Geochemistry; Prentice Hall: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Villalobos, M.; Trotz, M.A.; Leckie, J.O. Variability in Goethite Surface Site Density: Evidence from Proton and Carbonate Sorption. J. Colloid Interface Sci. 2003, 268, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Houben, G.; Kaufhold, S. Multi-Method Characterization of the Ferrihydrite to Goethite Transformation. Clay Miner. 2011, 46, 387–395. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties Reactions Occurrence and Uses; Wiley-VCH Verlag GmbH and Co. KGaA: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- Sheals, J.; Sjoberg, S.; Persson, P. Adsorption of Glyphosate on Goethite: Molecular Characterization of Surface Complexes. Environ. Sci. Technol. 2002, 36, 3090–3095. [Google Scholar] [CrossRef] [PubMed]
- Olsson, R.; Giesler, R.; Loring, J.S.; Persson, P. Adsorption, Desorption, and Surface-Promoted Hydrolysis of Glucose-1-Phosphate in Aqueous Goethite (Alpha-Feooh) Suspensions. Langmuir 2010, 26, 18760–18770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejedor-Tejedor, M.I.; Anderson, M.A. The Protonation of Phosphate on the Surface of Goethite as Studied by CIR-FTIR and Electrophoretic Mobility. Langmuir 1990, 6, 602–611. [Google Scholar] [CrossRef]
- Kim, J.; Li, W.; Philips, B.L.; Grey, C.P. Phosphate Adsorption on the Iron Oxyhydroxides Goethite (Alpha-FeOOH), Akaganeite (Beta-FeOOH), and Lepidocrocite (Gamma-FeOOH): A 31P NMR Study. Energy Environ. Sci. 2011, 4, 4298. [Google Scholar] [CrossRef]
- Loring, J.S.; Sandström, M.H.; Norén, K.; Persson, P. Rethinking Arsenate Coordination at the Surface of Goethite. Chem. Eur. J. 2009, 15, 5063–5072. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.D.; Kubicki, J.D. Molecular Orbital Theory Study on Surface Complex Structures of Phosphates to Iron Hydroxides: Calculation of Vibrational Frequencies and Adsorption Energies. Langmuir 2004, 20, 9249–9254. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, J.D.; Paul, K.W.; Kabalan, L.; Zhu, Q.; Mrozik, M.K.; Aryanpour, M.; Pierre-Louis, A.M.; Strongin, D.R. ATR–FTIR and Density Functional Theory Study of the Structures, Energetics, and Vibrational Spectra of Phosphate Adsorbed onto Goethite. Langmuir 2012, 28, 14573–14587. [Google Scholar] [CrossRef] [PubMed]
- Ganta, P.B.; Kühn, O.; Ahmed, A.A. QM/MM Molecular Dynamics Investigation of the Binding of Organic Phosphates to the 100 Diaspore Surface. Front. For. Glob. Chang. 2020, 3, 71. [Google Scholar] [CrossRef]
- Dultz, S.; Steinke, H.; Mikutta, R.; Woche, S.K.; Guggenberger, G. Impact of Organic Matter Types on Surface Charge and Aggregation of Goethite. Colloid Surf. A 2018, 554, 156–168. [Google Scholar] [CrossRef]
- Antelo, J.; Avena, M.; Fiol, S.; López, R.; Arce, F. Effects of pH and Ionic Strength on the Adsorption of Phosphate and Arsenate at the Goethite– Water Interface. J. Colloid Interface Sci. 2005, 285, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the Bet Equation Applicable to Microporous Adsorbents? In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 160, pp. 49–56. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Tempkin, M.I.; Pyzhev, V. Kinetics of Ammonia Synthesis on Promoted Iron Catalyst. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]
- Bolster, C.H.; Hornberger, G.M. On the Use of Linearized Langmuir Equations. Soil Sci. Soc. Am. J. 2007, 71, 1796–1806. [Google Scholar] [CrossRef]
- Rakovan, J.; Becker, U.; Hochella, M.F. Aspects of Goethite Surface Microtopography, Structure, Chemistry, and Reactivity. Am. Mineral. 1999, 84, 884–894. [Google Scholar] [CrossRef]
- Ognalaga, M.; Frossard, E.; Thomas, F. Glucose-1-Phosphate and Myo-Inositol Hexaphosphate Adsorption Mechanisms on Goethite. Soil Sci. Soc. Am. J. 1994, 332–337. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Gypser, S.; Freese, D.; Leinweber, P.; Kuehn, O. Molecular Level Picture of the Interplay between pH and Phosphate Binding at the Goethite–Water Interface. Phys. Chem. Chem. Phys. 2020. [Google Scholar] [CrossRef]
- Ganta, P.B.; Kühn, O.; Ahmed, A.A. Ab Initio Molecular Dynamics Simulations of the Interaction between Organic Phosphates and Goethite. Molecules 2021, 26, 160. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Ozboyaci, M.; Kokh, D.B.; Corni, S.; Wade, R.C. Modeling and Simulation of Protein–Surface Interactions: Achievements and Challenges. Q. Rev. Biophys. 2016, 49, e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubicki, J.D. (Ed.) Molecular Modeling of Geochemical Reactions; Wiley and Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- VandeVondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. Quickstep: Fast and Accurate Density Functional Calculations Using a Mixed Gaussian and Plane Waves Approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef] [Green Version]
- Krack, M. Pseudopotentials for H to Kr Optimized for Gradient-Corrected Exchange-Correlation Functionals. Theor. Chem. Acc. 2005, 114, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Phys. Chem. 2010, 132, 154104:1–154104:15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VandeVondele, J.; Hutter, J. Gaussian Basis Sets for Accurate Calculations on Molecular Systems in Gas and Condensed Phases. J. Chem. Phys. 2007, 127, 114105:1–114105:8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundy, C.J.; Balasubramanian, S.; Bagchi, K.; Hutter, J.; Kuo, A.S.I.; Laino, T.; VandeVondele, J. Frontiers in Simulation Technology (FIST). 2017. Available online: www.cp2k.org (accessed on 4 February 2021).
- Cygan, R.T.; Liang, J.J.; Kalinichev, A.G. Molecular Models of Hydroxide, Oxyhydroxide, and Clay Phases and the Development of a General Force Field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The Missing Term in Effective Pair Potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Zoete, V.; Cuendet, M.A.; Grosdidier, A.; Michielin, O. SwissParam: A Fast Force Field Generation Tool for Small Organic Molecules. J. Comput. Chem. 2011, 32, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Laino, T.; Mohamed, F.; Laio, A.; Parrinello, M. An Efficient Linear-Scaling Electrostatic Coupling for Treating Periodic Boundary Conditions in QM/MM Simulations. J. Chem. Theory Comput. 2006, 2, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical Sampling through Velocity Rescaling. J. Chem. Phys. 2007, 126, 014101:1–014101:6. [Google Scholar] [CrossRef] [Green Version]
- Boys, S.F.; Bernardi, F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies: Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Tellinghuisen, J.; Bolster, C.H. Least-Squares Analysis of Phosphorus Soil Sorption Data with Weighting from Variance Function Estimation: A Statistical Case for the Freundlich Isotherm. Environ. Sci. Technol. 2010, 44, 5029–5034. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Kühn, O.; Aziz, S.G.; Hilal, R.H.; Leinweber, P. How Soil Organic Matter Composition Controls Hexachlorobenzene-Soil-Interactions: Adsorption Isotherms and Quantum Chemical Modeling. Sci. Total Environ. 2014, 476, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Thiele-Bruhn, S.; Aziz, S.G.; Hilal, R.H.; Elroby, S.A.; Al-Youbi, A.O.; Leinweber, P.; Kühn, O. Interaction of Polar and Nonpolar Organic Pollutants with Soil Organic Matter: Sorption Experiments and Molecular Dynamics Simulation. Sci. Total Environ. 2015, 508, 276–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celi, L.; Lamacchia, S.; Marsan, F.A.; Barberis, E. Interaction of Inositol Hexaphosphate on Clays: Adsorption and Charging Phenomena. Soil Sci. 1999, 164, 574–585. [Google Scholar] [CrossRef]
- Martin, M.; Celi, L.; Barberis, E. Desorption and Plant Availability of Myo-Inositol Hexaphosphate Adsorbed on Goethite. Soil Sci. 2004, 169, 115–124. [Google Scholar] [CrossRef]
- Celi, L.; Barberis, E. Abiotic Reactions of Inositol Phosphates in Soil. In Inositol Phosphates: Linking Agriculture and the Environment; Turner, B.L., Richardson, A., Mullaney, E.J., Eds.; CABI Publishing: Wallingford, UK, 2007. [Google Scholar]
- Li, H.; Wan, B.; Yan, Y.; Zhang, Y.; Cheng, W.; Feng, X. Adsorption of Glycerophosphate on Goethite: A Macroscopic and Infrared Spectroscopic Study. J. Soil Sci. Plant Nutr. 2017, 181, 557–565. [Google Scholar] [CrossRef]
- Guzman, G.; Alcantara, E.; Barron, V.; Torrent, J. Phytoavailability of Phosphate Adsorbed on Ferrihydrite, Hematite, and Goethite. Plant Soil 1994, 159, 219–225. [Google Scholar] [CrossRef]
- Celi, L.; Presta, M.; Marsan, F.A.; Barberis, E. Effects of pH and Electrolytes on Inositol Hexaphosphate Interaction with Goethite. Soil Sci. Soc. Am. J. 2001, 65, 753–760. [Google Scholar] [CrossRef]
- Johnson, B.B.; Quill, E.; Angove, M.J. An Investigation of the Mode of Sorption of Inositol Hexaphosphate to Goethite. J. Colloid Interface Sci. 2012, 367, 436–442. [Google Scholar] [CrossRef]
- Xu, C.Y.; Li, J.Y.; Xu, R.K.; Hong, Z.N. Sorption of Organic Phosphates and Its Effects on Aggregation of Hematite Nanoparticles in Monovalent and Bivalent Solutions. Environ. Sci. Pollut. Res. 2017, 24, 7197–7207. [Google Scholar] [CrossRef]
- Abdala, D.B.; Northrup, P.A.; Arai, Y.; Sparks, D.L. Surface Loading Effects on Orthophosphate Surface Complexation at the Goethite/Water Interface as Examined by Extended X-Ray Absorption Fine Structure (EXAFS) Spectroscopy. J. Colloid Interface Sci. 2015, 437, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Persson, P.; Nilsson, N.; Sjöberg, S. Structure and Bonding of Orthophosphate Ions at the Iron Oxide–Aqueous Interface. J. Colloid Interface Sci. 1996, 177, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.; Flank, A.M.; Masion, A.; Bottero, J.Y.; Elmerich, P. Nucleation and Growth Mechanisms of Fe Oxyhydroxide in the Presence of PO4 Ions. 2. P K-Edge EXAFS Study. Langmuir 1997, 13, 1827–1834. [Google Scholar] [CrossRef]
- Tribe, L.; Kwon, K.D.; Trout, C.C.; Kubicki, J.D. Molecular Orbital Theory Study on Surface Complex Structures of Glyphosate on Goethite: Calculation of Vibrational Frequencies. Environ. Sci. Technol. 2006, 40, 3836–3841. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.; Andersson, T.; Nelson, H.; Sjöberg, S.; Giesler, R.; Lövgren, L. Surface Complexes of Monomethyl Phosphate Stabilized by Hydrogen Bonding on Goethite (Alpha-FeOOH) Nanoparticles. J. Colloid Interface Sci. 2012, 386, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.H.; Shang, C.; Zhu, J.; Chen, G.H. ATR-FTIR Investigation on the Complexation of Myo-Inositol Hexaphosphate with Aluminum Hydroxide. J. Colloid Interface Sci. 2006, 293, 296–302. [Google Scholar] [CrossRef] [PubMed]
- De Groot, C.J.; Golterman, H.L. On the Presence of Organic Phosphate in Some Camargue Sediments: Evidence for the Importance of Phytate. Hydrobiologia 1993, 252, 117–126. [Google Scholar] [CrossRef]




| Sorbate | Freundlich | Langmuir | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OP (μmol P) | 0.29 ± 0.08 | 0.57 ± 0.23 | 0.97 | 7.64 ± 2.36 | 0.001 ± 0.001 | 0.88 | 0.75 ± 0.16 | 0.49 ± 0.23 | 0.83 |
| GP (μmol P) | 0.19 ± 0.01 | 0.22 ± 0.02 | 0.98 | 1.20 ± 0.06 | 0.003 ± 0.001 | 0.81 | 0.13 ± 0.03 | 0.89 ± 0.30 | 0.92 |
| IHP (μmol P) | 0.07 ± 0.001 | 4.79 ± 0.02 | 0.98 | 8.35 ± 0.04 | 0.06 ± 0.002 | 0.92 | 0.52 ± 0.01 | 3104.43 ± 467.8 | 0.99 |
| OP (μmol) | 0.29 ± 0.08 | 0.57 ± 0.23 | 0.97 | 7.64 ± 2.36 | 0.001 ± 0.001 | 0.88 | 0.75 ± 0.16 | 0.49 ± 0.23 | 0.83 |
| GP (μmol) | 0.19 ± 0.01 | 0.22 ± 0.02 | 0.98 | 1.20 ± 0.06 | 0.003 ± 0.001 | 0.81 | 0.13 ± 0.03 | 0.89 ± 0.30 | 0.92 |
| IHP (μmol) | 0.07 ± 0.001 | 0.90 ± 0.002 | 0.98 | 1.39 ± 0.006 | 0.36 ± 0.0009 | 0.92 | 0.09 ± 0.0004 | 18,444.8 ± 148.6 | 0.99 |
| OP (mg) | 0.29 ± 0.07 | 0.14 ± 0.06 | 0.97 | 1.04 ± 0.4 | 0.01 ± 0.01 | 0.88 | 0.10 ± 0.02 | 3.6 ± 1.1 | 0.83 |
| GP (mg) | 0.20 ± 0.01 | 0.05 ± 0.003 | 0.98 | 0.21 ± 0.01 | 0.02 ± 0.004 | 0.81 | 0.02 ± 0.001 | 5.19 ± 1.53 | 0.92 |
| IHP (mg) | 0.07 ± 0.001 | 0.61 ± 0.001 | 0.98 | 0.92 ± 0.004 | 0.55 ± 0.01 | 0.92 | 0.06 ± 0.0002 | 7427.1 ± 112.6 | 0.99 |
| P | Motif | /bond (kcal mol) | Fe– (Å) | Fe–P (Å) |
|---|---|---|---|---|
| OP | M | –35 | 2.1 | 3.5 |
| B | –41 | 2.01 & 1.97 | 3.19 & 3.2 | |
| GP | M | –48 | 1.99 | 3.19 |
| B | –38 | 2.01 & 2.03 | 3.19 & 3.1 | |
| IHP | M(1) | –85 | 2.0 | 3.1 |
| M(2) | –69 | 2.03 | 3.2 | |
| 3M | –72 | 2.01 & 1.98 & 1.94 | 3.1 & 3.2 & 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganta, P.B.; Morshedizad, M.; Kühn, O.; Leinweber, P.; Ahmed, A.A. The Binding of Phosphorus Species at Goethite: A Joint Experimental and Theoretical Study. Minerals 2021, 11, 323. https://doi.org/10.3390/min11030323
Ganta PB, Morshedizad M, Kühn O, Leinweber P, Ahmed AA. The Binding of Phosphorus Species at Goethite: A Joint Experimental and Theoretical Study. Minerals. 2021; 11(3):323. https://doi.org/10.3390/min11030323
Chicago/Turabian StyleGanta, Prasanth B., Mohsen Morshedizad, Oliver Kühn, Peter Leinweber, and Ashour A. Ahmed. 2021. "The Binding of Phosphorus Species at Goethite: A Joint Experimental and Theoretical Study" Minerals 11, no. 3: 323. https://doi.org/10.3390/min11030323