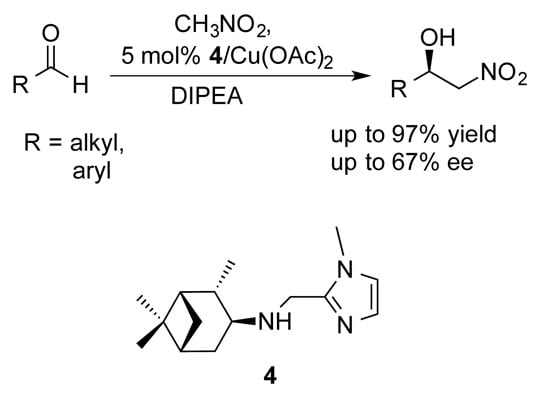
Cu (II)-Catalyzed Asymmetric Henry Reaction with a Novel C1-Symmetric Aminopinane-Derived Ligand
Abstract
:1. Introduction

2. Results and Discussion


| Entry a | Copper Salt | Solvent | T (°C) | Time (h) | DIPEA (mol %) | Yield (%) b | e.e. (%) c |
|---|---|---|---|---|---|---|---|
| 1 | CuCl2∙2H2O | THF | 4 | 20 | 100 | 90 | 53 |
| 2 | Cu(OAc)2∙H2O | THF | 4 | 20 | 100 | 52 | 52 |
| 3 | CuCl2∙2H2O | EtOH | 4 | 20 | 100 | 88 | 33 |
| 4 | Cu(OAc)2∙H2O | EtOH | 4 | 20 | 100 | 92 | 37 |
| 5 | Cu(OAc)2∙H2O | CH2Cl2 | 4 | 20 | 100 | 68 | 47 |
| 6 | Cu(OAc)2∙H2O | i-PrOH | 4 | 20 | 100 | 97 | 55 |
| 7 | Cu(OAc)2∙H2O | Et2O | 4 | 20 | 100 | 63 | 46 |
| 8 | Cu(OAc)2∙H2O | THF | −25 | 20 | 100 | <5 | n.d. d |
| 9 | Cu(OAc)2∙H2O | i-PrOH | −25 | 72 | 100 | 88 | 57 |
| 10 | Cu(OAc)2∙H2O | i-PrOH | 4 | 2 | 100 | 68 | 57 |
| 11 | Cu(OAc)2∙H2O | i-PrOH | 4 | 4 | 100 | 88 | 56 |
| 12 | Cu(OAc)2∙H2O | i-PrOH | 4 | 6 | 100 | 85 | 55 |
| 13 | Cu(OAc)2∙H2O | i-PrOH | 4 | 8 | 100 | 89 | 57 |
| 15 | Cu(OAc)2∙H2O | i-PrOH | 4 | 20 | 0 | 36 | 56 |
| 16 | Cu(OAc)2∙H2O | i-PrOH | 4 | 20 | 5 | 88 | 57 |
| 17 | Cu(OAc)2∙H2O | i-PrOH | 4 | 20 | 10 | 98 | 56 |
| Entry a | R | Aldehyde | Product | Yield (%) b | e.e. (%) c |
|---|---|---|---|---|---|
| 1 | n-Bu | 5a | 6a | 91 | 57 |
| 2 | i-Bu | 5b | 6b | 89 | 49 |
| 3 | t-Bu | 5c | 6c | 88 | 67 |
| 4 | i-Pr | 5d | 6d | 83 | 60 |
| 5 | n-nonyl | 5e | 6e | 75 | 55 |
| 6 | n-undecyl | 5f | 6f | 82 | 53 |
| 7 | trans-2-decenyl | 5g | 6g | 65 | 47 |
| 8 d | phenyl | 5h | 6h | 80 | 38 |
| 9 e | phenyl | 5h | 6h | 63 | 45 |
| 10 f | phenyl | 5h | 6h | 10 | 76 |
| Entry a | R | Product | Yield (%) b | syn/anti (%) c | ee (%) d |
|---|---|---|---|---|---|
| 1 | i-Bu | 7a | 93 | 58/42 | 54/38 |
| 2 | i-Pr | 7b | 94 | 81:19 | 66/34 |
3. Experimental Section
3.1. Preparation of Ligands
3.1.1. Synthesis of (E)-1-(1-Methyl-1H-imidazol-2-yl)-N-((1S,2S,3S,5R)-2,6,6-trimethylbicyclo[3.1.1]heptan-3-yl)methanimine (3)
3.1.2. Synthesis of (1S,2S,3S,5R)-2,6,6-Trimethyl-N-((1-methyl-1H-imidazol-2-yl)methyl)-bicyclo[3.1.1]heptan-3-amine (4)
3.2. General Procedure for the Enantioselective Henry Reaction
3.2.1. R-(−)-1-Nitro-2-hexanol (6a)
3.2.2. R-(+)-4-Methyl-1-nitropentan-2-ol (6b)
3.2.3. R-(−)-3,3-Dimethyl-1-nitrobutan-2-ol (6c)
3.2.4. R-(−)-3-Methyl-1-nitrobutan-2-ol (6d)
3.2.5. R-(−)-1-Nitroundecan-2-ol (6e)
3.2.6. R-(−)-1-Nitrotridecan-2-ol (6f)
3.2.7. R-(−)-(E)-1-Nitroundec-3-en-2-ol (6g)
3.2.8. (R)-(−)-1-Phenyl-2-nitroethanol (6h)
3.2.9. (2R,3R)-5-Methyl-2-nitrohexane-3-ol (7a)
3.2.10. (3R, 4R)-2-methyl-4-nitropentan-3-ol (7b)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ono, N. The Nitrogroup in Organic Synthesis; Wiley-VCH: New York, USA, 2001. [Google Scholar]
- Luzzio, F.A. The Henry reaction: Recent examples. Tetrahedron 2001, 57, 915–945. [Google Scholar] [CrossRef]
- Sasai, H.; Suzuki, T.; Arai, S.; Shibasaki, M. Basic character of rare earth metal alkoxides. Utilization in catalytic C-C-bond-forming reactions and catalytic asymmetric nitroaldol reactions. J. Am. Chem. Soc. 1992, 114, 4418–4420. [Google Scholar] [CrossRef]
- Trost, B.M.; Yeh, V.S.C.; Ito, H.; Bremeyer, N. Effect of ligand structure on zinc-catalyzed Henry reaction. Asymmertic synthesis of (−)-denopamine and (−)-arbutamine. Org. Lett. 2002, 4, 2621–2623. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Reddy, L.A.; Dwivedi, N.; Naram, J.; Swapna, R.; Malakondaiah, G.C.; Ravikumar, M.; Bhalerao, D.; Pratap, T.P.; Reddy, P.P.; et al. Diastereoselective synthesis of a core fragment of ritonavir and lopinavir. Tetrahedron Lett. 2011, 52, 6968–6970. [Google Scholar] [CrossRef]
- Guo, Z.L.; Deng, Y.Q.; Zhong, S.; Lu, G. Enantioselective synthesis of (R)-salmeterol employing an asymmetric Henry reaction as the key step. Tetrahedron: Asymmetry 2011, 22, 1395–1399. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, J.; Zhang, F.; Gong, Y. Synthesis of C1-symmetric chiral secondary diamines and their applications in the asymmetric copper(II)-catalyzed Henry (nitroaldol) reactions. J. Org. Chem. 2011, 76, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Uraguchi, D.; Nakamura, S.; Ooi, T. Catalytic asymmetric direct Henry reaction of ynals: Short synthesis of (2S,3R)-(+)-xestoaminol C and (−)-codonopsinines. Angew. Chem. Int. Ed. 2010, 49, 7562–7565. [Google Scholar] [CrossRef]
- Xu, K.; Lai, G.; Zha, Z.; Pan, S.; Chen, H.; Wang, Z. A highly anti-selective asymmetric Henry reaction catalyzed by a chiral copper complex: Applications to the syntheses of spisulisine and a pyrroloisoquinoline derivative. Chem. Eur. J. 2012, 18, 12357–12362. [Google Scholar] [CrossRef] [PubMed]
- Palomo, C.; Oiarbide, M.; Laso, A. Recent advances in catalytic asymmetric nitroaldol (Henry) reaction. Eur. J. Org. Chem. 2007, 2561–2574. [Google Scholar] [CrossRef]
- Boruwa, J.; Gogoi, N.; Saikia, P.P.; Barua, N.C. Catalytic asymmetric Henry reaction. Tetrahedron: Asymmetry 2006, 17, 3315–3326. [Google Scholar] [CrossRef]
- Alvarez-Casao, Y.; Marques-Lopez, E.; Herrera, R.P. Organocatalytic enantioselective Henry reaction. Symmetry 2011, 3, 220–245. [Google Scholar] [CrossRef] [Green Version]
- Ube, H.; Terada, M. Enantioselective Henry (nitroaldol) reaction catalyzed by axially chiral guanidines. Bioorg. Med. Chem. Lett. 2009, 19, 3895–3898. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, F.; Xue, F.; Lou, G.; Zhang, H.; Ma, S.; Duan, W.; Wang, W. Catalytic enantioselective Henry reaction of isatins: Application in concise synthesis of (S)-(−)-spirobrassinin. Chem. Eur. J. 2011, 17, 7791–7795. [Google Scholar] [CrossRef] [PubMed]
- Marcelli, T.; Haas, R.N. S.; Maarseveen, J.H.; Hiemstra, H. Asymmetric organocatalytic Henry reaction. Angew. Chem. Int. Ed. 2006, 45, 929–931. [Google Scholar] [CrossRef]
- Trost, B.; Yeh, V. A dinuclear Zn calalyst for the asymmetric nitroaldol (Henry) reaction. Angew. Chem. Int. Ed. 2002, 41, 861–863. [Google Scholar] [CrossRef]
- Lang, K.; Park, J.; Hong, S. Urea/transition-metal cooperative catalyst for anti-selective asymmetric nitroaldol reaction. Angew. Chem. Int. Ed. 2012, 51, 1620–1624. [Google Scholar] [CrossRef]
- Kogami, Y.; Nakajima, T.; Ikeno, T.; Yamada, T. Enantioselective Henry reaction catalyzed by salen-cobalt complexes. Synthesis 2004, 1947–1950. [Google Scholar]
- Zulauf, A.; Mallah, M.; Schulz, E. New chiral thiophene chromium complexes for asymmetric Henry reaction. J. Org. Chem. 2009, 74, 2242–2245. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, R.; Sidorowicz, L.; Skarzewski, J. Asymmetric nitroaldol reaction catalyzes by a chromium(III)-salen system. Tetrahedron: Asymmetry 2007, 18, 2581–2586. [Google Scholar] [CrossRef]
- Evans, D.A.; Seidel, D.; Rueping, M.; Lam, H.W.; Shaw, J.T.; Downey, C.W. A new copper acetate-bis(oxazoline)-catalyzed, enantioselective Henry reaction. J. Am. Chem. Soc. 2003, 125, 12692–12693. [Google Scholar] [CrossRef] [PubMed]
- Ginotra, S.K.; Singh, V.K. Enantioselective Henry reaction catalyzed by a C2-symmetric bis(oxazoline)- Cu(OAc)2∙H2O complex. Org. Biomol. Chem. 2007, 5, 3932–3937. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Kim, J.; Shin, Y.; Yeom, C.E.; Lee, J.E.; Hueon, T.; Kim, B.M. Heterogeneous asymmetric Henry reaction using a chiral bis(oxazoline)-copper complex immobilized in magnetically separable mesocellular mesoporous silica support. Tetrahedron: Asymmetry 2010, 21, 285–291. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Sidirowicz, L.; Skarzewski, J. Asymmetric Henry reaction catalyzed by chiral secondary diamine-copper(II) complexes. Tetrahedron: Asymmetry 2008, 19, 2310–2315. [Google Scholar] [CrossRef]
- Luo, M.; Yan, B. Enantioselective Henry reaction catalyzed cy chiral N-metal complexes containing R(+)/S(−)-α-ethylphenyl amines. Tetrahedron Lett. 2010, 51, 5577–5580. [Google Scholar] [CrossRef]
- Kodama, K.; Sugawara, K.; Hirose, T. Benzylidene acetal type bridged nucleic acids: Changes in properties upon cleavage of the bridged triggered by external stimuli. Chem. Eur. J. 2011, 17, 7918–7926. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, S.; Sevasankaran, D.; Singh, V.K. Enantioselective Henry reaction catalyzed by C2-symmetric chiral diamine-copper(II) complex. Org. Biomol. Chem. 2009, 7, 3156–3162. [Google Scholar] [CrossRef]
- Arai, T.; Watanabe, M.; Yanadisawa, A. Practical Asymmetric Henry reaction catalyzed by a chiral diamine- Cu(OAc)2 complex. Org. Lett. 2007, 9, 3595–3597. [Google Scholar] [CrossRef] [PubMed]
- Noole, A.; Lippur, K.; Metsala, A.; Lopp, M.; Kanger, T. Enantioselective Henry reaction catalyzed by Cu (II) salt and bipiperidine. J. Org. Chem. 2010, 75, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Chougnet, A.; Zhang, G.; Liu, K.; Haussinger, D.; Kagi, A.; Allmendinger, T.; Woggon, W.D. Diastereoselective and highly enantioselective Henry reactions using C1-symmertical copper(II) complexes. Adv. Synth. Catal. 2011, 353, 1797–1806. [Google Scholar] [CrossRef]
- Chunhong, Z.; Liu, F.; Gou, S. Application of chiral N,N'-dialkyl-1,2-cyclohexanediamine derivatives in asymmetric copper(II)- catalyzed Henry reactions. Tetrahedron: Asymmetry 2014, 25, 278–283. [Google Scholar] [CrossRef]
- Liu, F.; Gou, S.; Li, L. Asymmetric Henry reaction of aldehydes vith various nitroalkanes catalyzed by copper(II) complexes on novel chiral N-monoalkyl cyclohexane-1,2-diamines. Appl. Organometal. Chem. 2014, 28, 186–193. [Google Scholar] [CrossRef]
- Wei, Y.; Yao, L.; Zhang, B.; He, W.; Zhang, S. Novel Schiff base ligand derived from cinchona alkaloids for Cu(II)-catalyzed asymmetric Henry reaction. Tetrahedron 2011, 67, 8552–8558. [Google Scholar] [CrossRef]
- Boobalan, R.; Lee, G.H.; Chen, C. Copper complex of aminoisoborneol Schiff base Cu2(SBAIB-d)2: An efficient catalyst for direct catalytic asymmetric nitroaldol (Henry) reaction. Adv. Synth. Catal. 2012, 354, 2511–2520. [Google Scholar]
- Yang, W.; Liu, H.; Du, D.M. Efficient in situ three-component formation of chiral oxazoline-Schiff base copper(II) complexes: Towards combinatorial library of chiral catalysts for asymmetric Henry reaction. Org. Biomol. Chem. 2010, 8, 2956–2960. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Guo, F.; Zheng, Y.; Fang, Y.; Song, H.; Xu, K.; Wang, S.; Zha, Z.; Wang, Z. Highly enantioselective Henry reactions in water catalyzed by a copper tertiary amine complex and applied in the synthesis of (S)-N-trans-feruloylctopamine. Chem. Eur. J. 2011, 17, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.L.; Zhong, S.; Li, Y.B.; Lu, G. Chiral 1,1'-binaphthylazepine derived amino-alcohol catalyzed asymmetric Henry reaction. Tetrahedron: Asymmetry 2011, 22, 238–245. [Google Scholar] [CrossRef]
- Qin, D.D.; Yu, W.; Zhou, J.D.; Zhan, Y.C.; Ruan, Y.P.; Zhou, Z.H.; Chen, H.B. Syn- and enantioselective Henry reactions of aliphatic aldehydes and application to the synthesis of safingol. Chem. Eur. J. 2013, 19, 16541–16544. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Reddy, S.M.; Manisha, S.; Madan, C. Asymmetric Henry reaction catalyzed by a chiral Cu(II) complex: A facile enantioselective synthesis of (S)-2-nitro-1-arylethanols. Tetrahedron: Asymmetry 2011, 22, 530–535. [Google Scholar] [CrossRef]
- Blay, G.; Climent, E.; Fernandez, I.; Hernandez-Olmos, V.; Pedro, J.R. Enantioselective Henry reaction catalyzed with copper(II)-iminopyridine complexes. Tetrahedron: Asymmetry 2007, 18, 1603–1612. [Google Scholar] [CrossRef]
- Blay, G.; Domingo, L.R.; Hernandez-Olmos, V.; Pedro, J.R. New highly asymmetric Henry reaction catalyzed by CuII and a C1-symmetric aminopyridine ligand, and its application to the synthesis of miconazole. Chem. Eur. J. 2008, 14, 4725–4730. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.Q.; Qi, G.; Judeh, Z.M.A. Efficient asymmetric copper(I)-catalyzed Henry reaction using chiral N-alkyl-C1-tetrahydro-1,1'-bisisoquinolines. Eur. J. Org. Chem. 2011, 4892–4898. [Google Scholar]
- Ji, Y.Q.; Qi, G.; Judeh, Z.M.A. Catalytic anti-selective asymmetric Henry (nitroaldol) reaction catalyzed by Cu(I)-amine-imine complexes. Tetrahedron: Asymmetry 2011, 22, 2065–2070. [Google Scholar] [CrossRef]
- Blay, G.; Hernandez-Olmos, V.; Pedro, J.R. Synthesis of (S)-(+)-sotalol and R-(−)-isoproterenol via a catalytic enantioselective Henry reaction. Tetrahedron: Asymmetry 2010, 21, 578–581. [Google Scholar] [CrossRef]
- Jin, W.; Li, X.; Huang, Y.; Wu, F.; Wan, B. A highly effective bis(sulfonamide)-diamine ligand: A unique chiral skeleton for the enantioselective Cu-catalyzed Henry reaction. Chem. Eur. J. 2010, 16, 8259–8261. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Takashita, R.; Endo, Y.; Watanabe, M.; Yanagisawa, A. Asymmetric syn-selective Henry reaction catalyzed by the sulfonyldiamine-CuCl-pyridine system. J. Org. Chem. 2008, 73, 4903–4906. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gong, Y. Asymmetric copper(II)-catalysed nitroaldol (Henry) reactions utilizing a chiral C1-symmetric dinitrogen ligand. Eur. J. Org. Chem. 2011, 6092–6099. [Google Scholar] [CrossRef]
- Sanjeevakumar, N.; Periasamy, M. Highlly enantioselective Henry reaction catalyzed by a new chiral C2-symmetric N,N'-bis(isobornyl)ethylendiamine-copper complex. Tetrahedron: Asymmetry 2009, 20, 1842–1847. [Google Scholar] [CrossRef]
- Ni, B.; He, J. Highly asymmetric Henry reaction catalyzed by chiral copper(II) complexes. Tetrahedron Lett. 2013, 54, 462–465. [Google Scholar] [CrossRef]
- Sema, H.A.; Bez, G.; Karmakar, S. Asymmetric Henry reaction catalyzed by L-proline derivatives in the presence of Cu(OAc)2: Isolation and characterization of an in situ formed Cu(II) complex. Appl. Organometal. Chem. 2014, 28, 290–297. [Google Scholar] [CrossRef]
- Csillag, K.; Nemeth, L.; Martinek, T.A.; Szakonyi, Z.; Fulop, F. Stereoselective synthesis of pinane-type tridentate aminodiols and their application in the enantioselective addition of diethylzinc to benzaldehyde. Tetrahedron: Asymmetry 2012, 23, 144–150. [Google Scholar] [CrossRef]
- Binder, C.M.; Bautista, A.; Zaidlewicz, M.; Krzeminski, M.P.; Oliver, A.; Singaram, B. Dual stereoselectivity in the dialkylzinc reaction using (−)-β-pinene derived amino alcohol chiral auxiliaries. J. Org. Chem. 1999, 74, 2337–2343. [Google Scholar] [CrossRef]
- Cherng, Y.J.; Fang, J.M.; Lu, T.J. Pinane-type reagents for enantioselective reactions: Reduction of ketones and addition of diethylzinc to aldehydes. J. Org. Chem. 1999, 64, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Siyutkin, D.E.; Kucherenko, A.S.; Frolova, L.L.; Kuchin, A.V.; Zlotin, S.G. 2-Hydroxy-3-[(S)-prolinamido]pinanes as novel bifunctional organocatalysts for asymmetric aldol reactions in aqueous media. Tetrahedron: Asymmetry 2011, 22, 1320–1324. [Google Scholar] [CrossRef]
- Krzeminski, M.P.; Cwiklinska, M. Chiral terpene auxiliaries II. Spiroborate esters derived from α-pinene—new catalysts for asymmetric borane reduction of prochiral ketones. Tetrahedron Lett. 2011, 52, 3919–3921. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Brown, H.C. C2-symmetric N,N'-bis(terpenyl)ethylenediamines synthesis and application in the enantioselective nitroaldol reaction. RSC Adv. 2014, 4, 14264–14269. [Google Scholar] [CrossRef]
- Dhahagani, K.; Rajesh, J.; Kanan, R.; Rajagopal, G. Asymmetric Henry reaction of aldehydes catalyzed by recyclable MCM-41 supported copper(II) salen complex. Tetrahedron: Asymmetry 2011, 22, 857–865. [Google Scholar] [CrossRef]
- Sohtome, Y.; Hasimoto, Y.; Nagasawa, K. Diastereoselective and enantioselective Henry (nitroaldol) reaction utilizing a guanidine-thiourea bifunctional organocatalyst. Eur. J. Org. Chem. 2006, 13, 2894–2897. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the ligand 4 is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippova, L.; Stenstrøm, Y.; Hansen, T.V. Cu (II)-Catalyzed Asymmetric Henry Reaction with a Novel C1-Symmetric Aminopinane-Derived Ligand. Molecules 2015, 20, 6224-6236. https://doi.org/10.3390/molecules20046224
Filippova L, Stenstrøm Y, Hansen TV. Cu (II)-Catalyzed Asymmetric Henry Reaction with a Novel C1-Symmetric Aminopinane-Derived Ligand. Molecules. 2015; 20(4):6224-6236. https://doi.org/10.3390/molecules20046224
Chicago/Turabian StyleFilippova, Liudmila, Yngve Stenstrøm, and Trond Vidar Hansen. 2015. "Cu (II)-Catalyzed Asymmetric Henry Reaction with a Novel C1-Symmetric Aminopinane-Derived Ligand" Molecules 20, no. 4: 6224-6236. https://doi.org/10.3390/molecules20046224