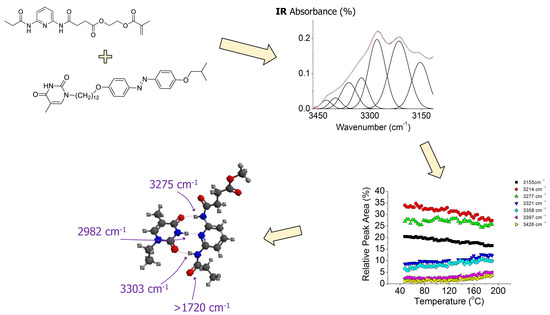
Molecular Recognition via Hydrogen Bonding in Supramolecular Complexes: A Fourier Transform Infrared Spectroscopy Study
Abstract
:1. Introduction
2. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lehn, J.M. Supramolecular chemistry. Science 1993, 260, 1762–1763. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, P.; Westhof, E. Rules governing the orientation of the 2’-hydroxyl group in RNA. J. Mol. Biol. 1997, 274, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Crowe, L.; Reid, D.; Crowe, J. Is trehalose special for preserving dry biomaterials? Biophys. J. 1996, 71, 2087–2093. [Google Scholar] [CrossRef] [Green Version]
- Wolkers, W.F.; Oliver, A.E.; Tablin, F.; Crowe, J.H. A Fourier-transform infrared spectroscopy study of sugar glasses. Carbohydr. Res. 2004, 339, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Nagamani, C.; Viswanathan, U.; Versek, C.; Tuominen, M.T.; Auerbach, S.M.; Thayumanavan, S. Importance of dynamic hydrogen bonds and reorientation barriers in proton transport. Chem. Commun. 2011, 47, 6638–6640. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Felipe, A.; Lu, Z.; Henderson, P.A.; Picken, S.J.; Norder, B.; Imrie, C.T.; Ribes-Greus, A. Synthesis and characterisation of side chain liquid crystal copolymers containing sulfonic acid groups. Polymer 2012, 53, 2604–2612. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Imrie, C.T.; Ribes-Greus, A. Study of structure formation in side-chain liquid crystal copolymers by variable temperature Fourier transform infrared spectroscopy. Ind. Eng. Chem. Res. 2013, 52, 8714–8721. [Google Scholar] [CrossRef]
- Vanti, L.; Alauddin, S.M.; Zaton, D.; Aripin, N.F.K.; Giacinti-Baschetti, M.; Imrie, C.T.; Ribes-Greus, A.; Martinez-Felipe, A. Ionically conducting and photoresponsive liquid crystalline terpolymers: Towards multifunctional polymer electrolytes. Eur. Polym. J. 2018. [Google Scholar] [CrossRef]
- Brienne, M.J.; Gabard, J.; Lehn, J.M.; Stibor, I. Macroscopic expression of molecular recognition-supramolecular liquid-crystalline phases induced by association of complementary heterocyclic components. Chem. Commun. 1989, 15, 1868–1870. [Google Scholar] [CrossRef]
- Lehn, J.M. Perspectives in supramolecular chemistry-from molecular recognition towards molecular information-processing and self-organization. Angew. Chem. Int. Ed. 1990, 29, 1304–1319. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular chemistry-molecular information and the design of supramolecular materials. Makromol. Chem. Macromol. Symp. 1993, 69, 1–17. [Google Scholar] [CrossRef]
- Berl, V.; Schmutz, M.; Krische, M.J.; Khoury, R.G.; Lehn, J.M. Supramolecular polymers generated from heterocomplementary monomers linked through multiple hydrogen-bonding arrays-formation, characterization, and properties. Chem. Eur. J. 2002, 8, 1227–1244. [Google Scholar] [CrossRef]
- Fouquey, C.; Lehn, J.M.; Levelut, A.M. Molecular recognition directed self-assembly of supramolecular liquid crystalline polymers from complementary chiral components. Adv. Mater. 1990, 2, 254–257. [Google Scholar] [CrossRef]
- Prins, L.J.; Reinhoudt, D.N.; Timmerman, P. Noncovalent synthesis using hydrogen bonding. Angew. Chem. Int. Ed. 2001, 40, 2382–2426. [Google Scholar] [CrossRef]
- Kolesnichenko, I.V.; Anslyn, E.V. Practical applications of supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 2385–2390. [Google Scholar] [CrossRef] [PubMed]
- Amabilino, D.B.; Smith, D.K.; Steed, J.W. Supramolecular materials. Chem. Soc. Rev. 2017, 46, 2404–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Frechet, J.M.J. Stabilization of a liquid-crystalline phase through noncovalent interaction with a polymer side-chain. Macromolecules 1989, 22, 3818–3819. [Google Scholar] [CrossRef]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional liquid-crystalline assemblies: Self-organized soft materials. Angew. Chem. Int. Ed. 2006, 45, 38–68. [Google Scholar] [CrossRef]
- Broer, D.J.; Bastiaansen, C.M.W.; Debije, M.G.; Schenning, A.P.H.J. Functional organic materials based on polymerized liquid-crystal monomers: Supramolecular hydrogen-bonded systems. Angew. Chem. Int. Ed. 2012, 51, 7102–7109. [Google Scholar] [CrossRef] [PubMed]
- Feringán, B.; Romero, P.; Serrano, J.L.; Gimenez, R.; Sierra, T. Supramolecular columnar liquid crystals formed by hydrogen bonding between a clicked star-shaped s-triazine and benzoic acids. Chem. Eur. J. 2015, 21, 8859–8866. [Google Scholar] [CrossRef] [PubMed]
- Concellón, A.; Schenning, A.P.H.J.; Romero, P.; Marcos, M.; Serrano, J.L. Size-selective adsorption in nanoporous polymers from coumarin photo-cross-linked columnar liquid crystals. Macromolecules 2018, 51, 2349–2358. [Google Scholar] [CrossRef]
- Gimeno, N.; Ros, B.; Serrano, J.L.; De la Fuente, M.R. Noncovalent interactions as a tool to design new bent-core liquid-crystal materials. Chem Mater. 2008, 20, 1262–1271. [Google Scholar] [CrossRef]
- Del Barrio, J.; Blasco, E.; Toprakcioglu, C.; Koutsioubas, A.; Scherman, O.A.; Oriol, L.; Sanchez-Somolinos, C. Self-assembly and photoinduced optical anisotropy in dendronized supramolecular azopolymers. Macromolecules 2014, 47, 897–906. [Google Scholar] [CrossRef]
- Del Barrio, J.; Blasco, E.; Oriol, L.; Alcala, R.; Sanchez-Somolinos, C. Diblock copolymerazobenzene complexes through hydrogen bonding: Self-assembly and stable photoinduced optical anisotropy. J. Polym. Sci. Pol. Chem. 2013, 51, 1716–1725. [Google Scholar] [CrossRef]
- Vera, F.; Almuzara, C.; Orera, I.; Barbera, J.; Oriol, L.; Serrano, J.L.; Sierra, T. Side-chain supramolecular polymers with induced supramolecular chirality through H-bonding interactions. J. Polym. Sci. Pol. Chem. 2008, 46, 5528–5541. [Google Scholar] [CrossRef]
- Yao, M.; Chen, X.S.; Dong, L.; Wan, X.Y.; Xian, Y.P.; Yao, D.S.; Hu, J.S.; Tian, M. Synthesis and properties of new non-symmetric liquid crystal dimers containing mandelic acid and cyano group. Liq. Cryst. 2018, 45, 931–941. [Google Scholar] [CrossRef]
- Jansze, S.M.; Martinez-Felipe, A.; Storey, J.M.D.; Marcelis, A.T.M.; Imrie, C.T. A Twist-Bend Nematic Phase Driven by Hydrogen Bonding. Angew. Chem. Int. Ed. 2015, 54, 643–646. [Google Scholar] [CrossRef]
- Paterson, D.A.; Martinez-Felipe, A.; Jansze, S.M.; Marcelis, A.T.M.; Storey, J.M.D.; Imrie, C.T. New insights into the liquid crystal behaviour of hydrogen-bonded mixtures provided by temperature-dependent FTIR spectroscopy. Liq. Cryst. 2015, 5–6, 928–939. [Google Scholar] [CrossRef]
- Walker, R.; Pociecha, D.; Abberley, J.P.; Martinez-Felipe, A.; Paterson, D.A.; Forsyth, E.; Lawrence, G.B.; Henderson, P.A.; Storey, J.M.D.; Gorecka, E.; et al. Spontaneous chirality through mixing achiral components: A twist-bend nematic phase driven by hydrogen-bonding between unlike components. Chem. Commun. 2018, 54, 3383–3386. [Google Scholar] [CrossRef] [PubMed]
- Concellón, A.; Blasco, E.; Martinez-Felipe, A.; Martínez, J.L.; Sícs, I.; Ezquerra, T.A.; Nogales, A.; Pinol, M.; Oriol, L. Light-responsive self-assembled materials by supramolecular post-functionalization via hydrogen bonding of amphiphilic block copolymers. Macromolecules 2016, 49, 7825–7836. [Google Scholar] [CrossRef]
- Concellón, A.; Blasco, E.; Pinol, M.; Oriol, L.; Diez, I.; Berges, C.; Sanchez-Somolinos, C.; Alcala, R. Photoresponsive polymers and block copolymers by molecular recognition based on multiple hydrogen bonds. J. Polym. Sci. Pol. Chem. 2014, 52, 3173–3184. [Google Scholar] [CrossRef] [Green Version]
- Concellon, A.; Claveria-Gimeno, R.; Velazquez-Campoy, A.; Abian, O.; Pinol, M.; Oriol, L. Polymeric micelles from block copolymers containing 2,6-diacylaminopyridine units for encapsulation of hydrophobic drugs. RSC Adv. 2106, 6, 24066–24075. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Cook, A.G.; Wallage, M.J.; Imrie, C.T. Hydrogen bonding and liquid crystallinity of low molar mass and polymeric mesogens containing benzoic acids: A variable temperature Fourier transform infrared spectroscopic study. Phase Trans. 2014, 87, 1191–1210. [Google Scholar] [CrossRef]
- Sundaram, S.; Jayaprakasam, R.; Dhandapani, M.; Senthil, T.S.; Vijayakumar, V.N. Theoretical (DFT) and experimental studies on multiple hydrogen bonded liquid crystals comprising between aliphatic and aromatic acids. J. Mol. Liq. 2017, 243, 14–21. [Google Scholar] [CrossRef]
- Abdy, M.J.; Murdoch, A.; Martinez-Felipe, A. New insights into the role of hydrogen bonding on the liquid crystal behaviour of 4-alkoxybenzoic acids: A detailed IR spectroscopy study. Liq. Cryst. 2016, 43, 2191–2207. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Cook, A.G.; Abberley, J.P.; Walker, R.; Storey, J.M.D.; Imrie, C.T. An FT-IR spectroscopic study of the role of hydrogen bonding in the formation of liquid crystallinity for mixtures containing bipyridines and 4-pentyloxybenzoic acid. RSC Adv. 2016, 6, 108164–108179. [Google Scholar] [CrossRef]
- Odinokov, S.E.; Iogansen, A.V. Torsional gamma-(OH) vibrations, fermi resonance 2gamma-(OH)--NU-isotopic effects in IR-spectra of h-complexes of carboxylic-acids with strong bases. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1972, A28, 2343–2350. [Google Scholar] [CrossRef]
- Huang, C.; Wu, P.; Su, W.; Zhu, C.; Kuo, S. Stimuli-responsive supramolecular materials: Photo-tunable properties and molecular recognition behaviour. Polym. Chem. 2016, 7, 795–806. [Google Scholar] [CrossRef]
- Gilli, P.; Gilli, R. Hydrogen bond models and theories: The dual hydrogen bond model and its consequences. J. Mol. Struct. 2010, 972, 2–10. [Google Scholar] [CrossRef]
- Cleland, W.W.; Kreevoy, M.M. Low-barrier hydrogen-bonds and enzymatic catalysis. Science 1994, 264, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Wilson, P.G.; Fujishima, A.; Frechet, J.M.J. Hydrogen-bonded liquid-crystals-a novel mesogen incorporating nonmesogenic 4,4′-bipyridine through selective recognition between hydrogen-bonding donor and acceptor. Chem. Lett. 1990, 11, 2003–2006. [Google Scholar] [CrossRef]
- Johnson, S.L.; Rumon, K.A. Infrared spectra of solid 1–1 pyridine-benzoic acid complexes. nature of hydrogen bond as a function of acid-base levels in complex. J. Phys. Chem. 1965, 69, 74–86. [Google Scholar] [CrossRef]
- Xu, H.; Kang, N.; Xie, P.; Zhang, R.B. A new insight into the hydrogen-bonded liquid crystals built from carboxylic acids and pyridyl moieties. Mol. Cryst. Liq. Cryst. 2002, 373, 119–126. [Google Scholar] [CrossRef]
- Fonseca, J.M.S.; Santos, L.M.N.B.F.; Monte, M.J.S. Thermodynamic study of 4-n-alkyloxybenzoic acids. J. Chem. Eng. Data 2010, 55, 2238–2245. [Google Scholar] [CrossRef]
- Kato, T.; Kihara, H.; Ujiie, S.; Uryu, T.; Frechet, J.M.J. Structures and properties of supramolecular liquid-crystalline side-chain polymers built through intermolecular hydrogen bonds. Macromolecules 1996, 29, 8734–8739. [Google Scholar] [CrossRef]
- Kato, T.; Kihara, H.; Uryu, T.; Fujishima, A.; Frechet, J.M.J. Molecular self-assembly of liquid-crystalline side-chain polymers through intermolecular hydrogen-bonding-polymeric complexes built from a polyacrylate and stilbazoles. Macromolecules 1992, 25, 6836–6841. [Google Scholar] [CrossRef]
- Pourcain, C.B. An infrared study of hydrogen bond stability in benzoic acid-trans-1,2-bis(4-pyridyl)ethylene complexes in the solid state and the implications for the design of supramolecular polymers. J. Mater. Chem. 1999, 9, 2727–2730. [Google Scholar] [CrossRef]
- Lee, J.Y.; Painter, P.C.; Coleman, M.M. Hydrogen-bonding in polymer blends. 4. blends involving polymers containing methacrylic-acid and vinylpyridine groups. Macromolecules 1988, 21, 954–960. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Imrie, C.T. The role of hydrogen bonding in the phase behaviour of supramolecular liquid crystal dimers. J. Mol. Struct. 2015, 1100, 429–437. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds DAP, tAZOi, dAZOi, DAP+ tAZOi and DAP+dAZOi are available from the authors. |







| Dimers (and DAP Trimer) | |||
| dAZOi-2HB-sym | dAZOi-2HB-as | ||
| 116.91 | 60.23 | ||
| tAZOi-2HB-sym | tAZOi-2HB-sym(alt) | tAZOi-2HB-as | tAZOi-1HB-as |
| 76.58 | 70.81 | 73.15 | 43.01 |
| DAP2-4HB-sym | DAP3-2HB-as | DAP2-2HB-sym | |
| 118.21 | 129.45 | 83.35 | |
| Complexes | |||
| DAP●tAZOi-3HB | DAP●dAZOi-2HB | ||
| 92.92 | 89.66 | ||
| Pyr●tAZOi-1HB | Pyr●dAZOi-1HB | ||
| 62.83 | 74.82 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Felipe, A.; Brebner, F.; Zaton, D.; Concellon, A.; Ahmadi, S.; Piñol, M.; Oriol, L. Molecular Recognition via Hydrogen Bonding in Supramolecular Complexes: A Fourier Transform Infrared Spectroscopy Study. Molecules 2018, 23, 2278. https://doi.org/10.3390/molecules23092278
Martinez-Felipe A, Brebner F, Zaton D, Concellon A, Ahmadi S, Piñol M, Oriol L. Molecular Recognition via Hydrogen Bonding in Supramolecular Complexes: A Fourier Transform Infrared Spectroscopy Study. Molecules. 2018; 23(9):2278. https://doi.org/10.3390/molecules23092278
Chicago/Turabian StyleMartinez-Felipe, Alfonso, Fraser Brebner, Daniel Zaton, Alberto Concellon, Sara Ahmadi, Milagros Piñol, and Luis Oriol. 2018. "Molecular Recognition via Hydrogen Bonding in Supramolecular Complexes: A Fourier Transform Infrared Spectroscopy Study" Molecules 23, no. 9: 2278. https://doi.org/10.3390/molecules23092278