A Charge-Transfer Salt Based on Ferrocene/Ferrocenium Pairs and Keggin-Type Polyoxometalates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Infrared Spectroscopy
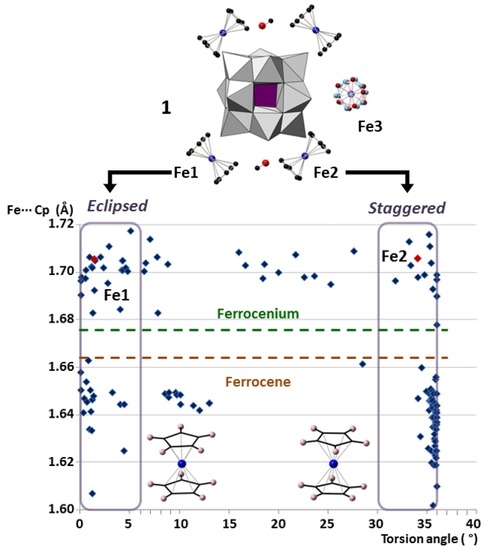
2.2. Crystal Structure
2.3. Diffuse Reflectance UV-Vis Spectroscopy
2.4. 57Fe Mössbauer Spectroscopy
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of [Fe(Cp)2]4[SiW12O40]·[Fe(Cp)2]·2CH3OH (1)
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| 1 | ||||||||||||
| W1 | W2 | W3 | W4 | W5 | W6 | |||||||
| Ot | O1 | 1.670 (5) | O2 | 1.655 (6) | O3 | 1.667 (6) | O4 | 1.658 (6) | O5 | 1.669 (7) | O6 | 1.666 (6) |
| Ob | O7 | 1.872 (8) | O12 | 1.891 (6) | O14 | 1.870 (6) | O16 | 1.885 (8) | O16 | 1.894 (6) | O17 | 1.885 (7) |
| O10 | 1.880 (8) | O7 | 1.904 (7) | O8 | 1.885 (8) | O11 | 1.892 (7) | O15 | 1.893 (6) | O13 | 1.891 (7) | |
| O11 | 1.887 (8) | O13 | 1.909 (6) | O9 | 1.891 (6) | O18 | 1.895 (8) | O17 | 1.900 (6) | O10 | 1.899 (7) | |
| O9 | 1.888 (7) | O8 | 1.913 (8) | O15 | 1.900 (7) | O14 | 1.896 (7) | O12 | 1.905 (6) | O18 | 1.900 (8) | |
| Oc | O22 | 2.372 (9) | O19 | 2.367 (11) | O19 | 2.344 (10) | O22 | 2.323 (10) | O20 | 2.348 (10) | O22 | 2.360 (11) |
| [SiW12O40]4− | 1 | Optimized [30] | ||||||||||
| Range | Average | |||||||||||
| W–Oc | 2.323–2.461 | 2.385 | 2.325 | |||||||||
| W–Ob | 1.870–1.913 | 1.893 | 1.916 | |||||||||
| W–Ot | 1.655–1.670 | 1.664 | 1.743 | |||||||||
| Si–Oc | 1.591–1.705 | 1.644 | 1.667 | |||||||||
| W···Si | 3.516–3.541 | 3.529 | 3.588 | |||||||||
| W···Wtrans | 7.032–7.082 | 7.057 | 7.172 | |||||||||
| O···Otrans | 10.364–10.421 | 10.386 | 10.619 | |||||||||
Appendix B
| Refcode | Fe–C | Mean | Cg···Cg′ | Fe···Cg | T | OS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFALAV | 2.074 | 2.076 | 2.071 | 2.065 | 2.071 | 2.075 | 2.086 | 2.088 | 2.077 | 2.071 | 2.075 | 3.395 | 1.697 | 1.698 | 18.844 | III |
| AFALID | 2.098 | 2.107 | 2.081 | 2.081 | 2.107 | 2.081 | 2.081 | 2.107 | 2.098 | 2.107 | 2.095 | 3.422 | 1.711 | 1.711 | 35.251 | III |
| AFALID01 | 2.081 | 2.093 | 2.068 | 2.068 | 2.093 | 2.068 | 2.068 | 2.093 | 2.081 | 2.093 | 2.081 | 3.408 | 1.704 | 1.704 | 35.423 | III |
| AFALOJ | 2.086 | 2.074 | 2.072 | 2.094 | 2.076 | 2.078 | 2.079 | 2.081 | 2.098 | 2.104 | 2.084 | 3.406 | 1.704 | 1.702 | 17.247 | III |
| AGEFEX | 2.06 | 2.027 | 2.039 | 2.056 | 2.046 | 2.066 | 2.051 | 2.03 | 2.077 | 2.047 | 2.050 | 3.407 | 1.702 | 1.706 | 5.975 | III |
| BEJLOR (Fe1) | 2.093 | 2.065 | 2.028 | 2.009 | 2.038 | 2.058 | 2.003 | 2.043 | 2.038 | 2.062 | 2.044 | 3.293 | 1.650 | 1.646 | 1.615 | II |
| BEJLOR (Fe2) | 2.038 | 2.051 | 2.064 | 2.065 | 2.049 | 1.986 | 2.078 | 2.087 | 2.058 | 2.003 | 2.048 | 3.305 | 1.658 | 1.650 | 0.369 | II |
| COTPUW02 | 2.056 | 2.046 | 2.103 | 2.046 | 2.056 | 2.07 | 2.073 | 2.087 | 2.073 | 2.07 | 2.068 | 3.394 | 1.698 | 1.697 | 0 | III |
| DOMZEM | 2.12 | 2.115 | 2.085 | 2.066 | 2.08 | 2.064 | 2.074 | 2.087 | 2.111 | 2.102 | 2.090 | 3.41 | 1.707 | 1.703 | 3.723 | III |
| DOQHOG | 1.985 | 1.991 | 1.959 | 1.934 | 2.01 | 2.01 | 1.934 | 1.959 | 1.991 | 1.985 | 1.976 | 3.118 | 1.559 | 1.559 | 0 | II |
| FANNUE | 2.01 | 2.01 | 2.015 | 2.01 | 2.01 | 2.015 | 2.01 | 2.01 | 2.01 | 2.01 | 2.011 | 3.275 | 1.637 | 1.637 | 34.704 | II |
| FANNUE01 | 2.001 | 2.005 | 2.054 | 2.005 | 2.001 | 2.054 | 2.005 | 2.001 | 2.001 | 2.005 | 2.013 | 3.221 | 1.610 | 1.610 | 34.268 | II |
| FEHYAS02 | 2.029 | 2.044 | 2.049 | 2.037 | 2.033 | 2.025 | 2.042 | 2.024 | 2.019 | 2.024 | 2.033 | 3.283 | 1.644 | 1.639 | 0.241 | II |
| FEHYAS03 | 2.04 | 2.047 | 2.044 | 2.037 | 2.045 | 2.036 | 2.043 | 2.037 | 2.03 | 2.024 | 2.038 | 3.291 | 1.647 | 1.644 | 0.622 | II |
| FEHYAS04 | 1.995 | 2.025 | 2.047 | 2.024 | 2.01 | 2.02 | 1.999 | 2.017 | 1.99 | 1.965 | 2.007 | 3.263 | 1.643 | 1.625 | 1.051 | II |
| FEOCAS | 2.074 | 2.062 | 2.063 | 2.052 | 2.065 | 2.066 | 2.064 | 2.068 | 2.078 | 2.083 | 2.067 | 3.403 | 1.699 | 1.705 | 2.783 | III |
| FERCBI10 | 2.068 | 2.073 | 2.073 | 2.114 | 2.086 | 2.065 | 2.09 | 2.071 | 2.054 | 2.062 | 2.076 | 3.404 | 1.706 | 1.699 | 0.487 | III |
| FERCBI11 | 2.109 | 2.086 | 2.063 | 2.073 | 2.105 | 2.102 | 2.094 | 2.068 | 2.085 | 2.107 | 2.089 | 3.412 | 1.705 | 1.708 | 2.391 | III |
| FEROCE16 (Fe1) | 2.043 | 2.038 | 2.046 | 2.051 | 2.049 | 2.05 | 2.045 | 2.048 | 2.048 | 2.048 | 2.047 | 3.297 | 1.648 | 1.650 | 8.735 | II |
| FEROCE16 (Fe2) | 2.041 | 2.045 | 2.053 | 2.048 | 2.046 | 2.046 | 2.048 | 2.045 | 2.042 | 2.044 | 2.046 | 3.295 | 1.649 | 1.646 | 9.023 | II |
| FEROCE17 (Fe1) | 2.038 | 2.035 | 2.041 | 2.048 | 2.044 | 2.049 | 2.041 | 2.048 | 2.044 | 2.047 | 2.043 | 3.299 | 1.648 | 1.651 | 9.118 | II |
| FEROCE17 (Fe2) | 2.035 | 2.043 | 2.05 | 2.046 | 2.04 | 2.042 | 2.048 | 2.042 | 2.038 | 2.045 | 2.043 | 3.298 | 1.65 | 1.649 | 9.512 | II |
| FEROCE18 (Fe1) | 2.034 | 2.035 | 2.039 | 2.043 | 2.045 | 2.05 | 2.043 | 2.046 | 2.041 | 2.043 | 2.042 | 3.297 | 1.647 | 1.650 | 9.837 | II |
| FEROCE18 (Fe2) | 2.027 | 2.039 | 2.052 | 2.045 | 2.036 | 2.043 | 2.046 | 2.043 | 2.034 | 2.046 | 2.041 | 3.296 | 1.648 | 1.649 | 10.283 | II |
| FEROCE24 | 2.055 | 2.051 | 2.063 | 2.063 | 2.051 | 2.054 | 2.052 | 2.052 | 2.054 | 2.062 | 2.056 | 3.316 | 1.655 | 1.661 | 0.069 | II |
| FEROCE26 | 2.012 | 2.035 | 2.042 | 2.023 | 2.005 | 2.023 | 2.005 | 2.012 | 2.035 | 2.042 | 2.023 | 3.287 | 1.644 | 1.644 | 35.998 | II |
| FEROCE27 | 2.009 | 2.012 | 2.031 | 2.039 | 2.026 | 2.031 | 2.039 | 2.026 | 2.009 | 2.012 | 2.023 | 3.277 | 1.639 | 1.639 | 35.983 | II |
| FEROCE35 | 2.031 | 2.01 | 2.005 | 2.017 | 2.022 | 2.017 | 2.022 | 2.031 | 2.01 | 2.005 | 2.017 | 3.238 | 1.619 | 1.619 | 35.735 | II |
| FEROCE36 | 2.038 | 2.011 | 2.007 | 2.016 | 2.024 | 2.016 | 2.024 | 2.038 | 2.011 | 2.007 | 2.019 | 3.239 | 1.620 | 1.620 | 35.44 | II |
| FEROCE37 | 2.041 | 2.016 | 2.009 | 2.018 | 2.033 | 2.018 | 2.033 | 2.041 | 2.016 | 2.009 | 2.023 | 3.244 | 1.622 | 1.622 | 35.585 | II |
| FEROCE38 | 2.032 | 2.012 | 2.008 | 2.017 | 2.034 | 2.017 | 2.034 | 2.032 | 2.012 | 2.008 | 2.021 | 3.238 | 1.619 | 1.619 | 35.778 | II |
| FEROCE39 | 2.041 | 2.011 | 2.016 | 2.016 | 2.035 | 2.016 | 2.035 | 2.041 | 2.011 | 2.016 | 2.024 | 3.247 | 1.623 | 1.623 | 35.762 | II |
| FEROCE40 | 2.036 | 2.015 | 2.016 | 2.021 | 2.033 | 2.021 | 2.033 | 2.036 | 2.015 | 2.016 | 2.024 | 3.249 | 1.625 | 1.625 | 35.833 | II |
| FEROCE41 | 2.037 | 2.018 | 2.014 | 2.021 | 2.035 | 2.021 | 2.035 | 2.037 | 2.018 | 2.014 | 2.025 | 3.251 | 1.625 | 1.625 | 35.731 | II |
| FEROCE42 | 2.026 | 2.052 | 2.021 | 2.012 | 2.019 | 2.021 | 2.052 | 2.026 | 2.019 | 2.012 | 2.026 | 3.252 | 1.626 | 1.626 | 34.943 | II |
| FEROCE43 | 2.031 | 2.017 | 2.017 | 2.02 | 2.035 | 2.02 | 2.035 | 2.031 | 2.017 | 2.017 | 2.024 | 3.247 | 1.624 | 1.624 | 35.825 | II |
| FEROCE44 | 2.04 | 2.022 | 2.018 | 2.022 | 2.036 | 2.022 | 2.036 | 2.04 | 2.022 | 2.018 | 2.028 | 3.258 | 1.629 | 1.629 | 35.816 | II |
| FEROCE45 | 2.044 | 2.021 | 2.018 | 2.028 | 2.041 | 2.028 | 2.041 | 2.044 | 2.021 | 2.018 | 2.030 | 3.26 | 1.630 | 1.630 | 35.881 | II |
| FEROCE46 | 2.044 | 2.021 | 2.02 | 2.026 | 2.045 | 2.026 | 2.045 | 2.044 | 2.021 | 2.02 | 2.031 | 3.264 | 1.632 | 1.632 | 35.498 | II |
| FEROCE47 | 2.045 | 2.025 | 2.024 | 2.027 | 2.036 | 2.027 | 2.036 | 2.045 | 2.025 | 2.024 | 2.031 | 3.272 | 1.636 | 1.636 | 35.870 | II |
| FEROCE48 | 2.044 | 2.024 | 2.029 | 2.032 | 2.037 | 2.032 | 2.037 | 2.044 | 2.024 | 2.029 | 2.033 | 3.271 | 1.636 | 1.636 | 35.723 | II |
| FEROCE49 | 2.040 | 2.033 | 2.027 | 2.02 | 2.034 | 2.020 | 2.034 | 2.04 | 2.033 | 2.027 | 2.031 | 3.270 | 1.635 | 1.635 | 35.471 | II |
| FEROCE50 | 2.053 | 2.028 | 2.036 | 2.023 | 2.044 | 2.023 | 2.044 | 2.053 | 2.028 | 2.036 | 2.037 | 3.277 | 1.639 | 1.639 | 35.602 | II |
| FEROCE51 | 2.054 | 2.026 | 2.026 | 2.03 | 2.051 | 2.026 | 2.03 | 2.051 | 2.054 | 2.026 | 2.037 | 3.282 | 1.641 | 1.641 | 35.753 | II |
| FEROCE52 | 2.045 | 2.029 | 2.028 | 2.032 | 2.049 | 2.032 | 2.049 | 2.045 | 2.029 | 2.028 | 2.037 | 3.285 | 1.642 | 1.642 | 35.794 | II |
| FEROCE53 | 2.052 | 2.024 | 2.027 | 2.031 | 2.046 | 2.027 | 2.031 | 2.046 | 2.052 | 2.024 | 2.036 | 3.282 | 1.641 | 1.641 | 35.743 | II |
| FEROCE54 | 2.051 | 2.028 | 2.022 | 2.025 | 2.042 | 2.025 | 2.042 | 2.051 | 2.028 | 2.022 | 2.034 | 3.288 | 1.644 | 1.644 | 35.784 | II |
| FEROCE55 | 2.073 | 2.016 | 2.017 | 2.011 | 2.06 | 2.017 | 2.011 | 2.06 | 2.073 | 2.016 | 2.035 | 3.292 | 1.646 | 1.646 | 34.983 | II |
| FEROCE56 | 2.061 | 2.025 | 2.016 | 2.018 | 2.036 | 2.016 | 2.018 | 2.036 | 2.061 | 2.025 | 2.031 | 3.293 | 1.646 | 1.646 | 34.804 | II |
| FEROCE57 | 2.062 | 2.018 | 2.019 | 2.015 | 2.028 | 2.019 | 2.015 | 2.028 | 2.062 | 2.018 | 2.028 | 3.292 | 1.646 | 1.646 | 34.976 | II |
| FEROCE58 | 2.060 | 2.022 | 2.021 | 2.013 | 2.022 | 2.021 | 2.013 | 2.022 | 2.06 | 2.022 | 2.028 | 3.294 | 1.647 | 1.647 | 34.540 | II |
| FEROCE59 | 2.049 | 2.04 | 2.009 | 2.030 | 2.034 | 2.009 | 2.030 | 2.034 | 2.049 | 2.040 | 2.032 | 3.301 | 1.651 | 1.651 | 35.456 | II |
| FEROCE60 | 2.059 | 1.981 | 2.009 | 2.033 | 2.085 | 2.009 | 1.981 | 2.059 | 2.085 | 2.033 | 2.033 | 3.299 | 1.649 | 1.649 | 35.317 | II |
| FEROCE61 | 2.011 | 2.072 | 2.072 | 1.970 | 2.036 | 1.970 | 2.036 | 2.011 | 2.072 | 2.072 | 2.032 | 3.297 | 1.649 | 1.649 | 35.955 | II |
| FEROCE62 | 2.024 | 2.017 | 1.982 | 2.067 | 2.073 | 1.982 | 2.017 | 2.024 | 2.073 | 2.067 | 2.033 | 3.297 | 1.649 | 1.649 | 35.283 | II |
| FEROCE63 | 2.030 | 2.038 | 1.995 | 2.027 | 2.052 | 1.995 | 2.038 | 2.030 | 2.052 | 2.027 | 2.028 | 3.287 | 1.643 | 1.643 | 35.361 | II |
| FEROCE64 | 2.032 | 2.042 | 2.025 | 2.026 | 2.039 | 2.025 | 2.042 | 2.032 | 2.039 | 2.026 | 2.033 | 3.294 | 1.647 | 1.647 | 35.406 | II |
| FEROCE65 | 2.031 | 2.052 | 2.044 | 2.046 | 2.024 | 2.046 | 2.024 | 2.031 | 2.052 | 2.044 | 2.039 | 3.292 | 1.646 | 1.646 | 35.621 | II |
| FEROCE66 | 2.031 | 2.026 | 2.001 | 2.042 | 2.033 | 2.001 | 2.026 | 2.031 | 2.033 | 2.042 | 2.027 | 3.279 | 1.639 | 1.639 | 35.444 | II |
| FEROCE67 | 2.055 | 2.018 | 2.011 | 2.016 | 2.028 | 2.011 | 2.016 | 2.028 | 2.055 | 2.018 | 2.026 | 3.312 | 1.656 | 1.656 | 34.620 | II |
| FEROCE68 | 2.065 | 2.023 | 2.014 | 2.022 | 2.037 | 2.014 | 2.022 | 2.037 | 2.065 | 2.023 | 2.032 | 3.293 | 1.647 | 1.647 | 34.804 | II |
| FEROCE69 | 2.045 | 2.030 | 2.011 | 2.028 | 2.029 | 2.011 | 2.028 | 2.029 | 2.045 | 2.03 | 2.029 | 3.300 | 1.650 | 1.650 | 35.185 | II |
| FEROCE70 | 2.044 | 2.019 | 2.035 | 2.017 | 2.047 | 2.035 | 2.019 | 2.044 | 2.047 | 2.017 | 2.032 | 3.299 | 1.649 | 1.649 | 35.774 | II |
| FEROCE71 | 2.047 | 2.032 | 2.019 | 2.028 | 2.023 | 2.019 | 2.028 | 2.023 | 2.047 | 2.032 | 2.030 | 3.300 | 1.650 | 1.650 | 35.510 | II |
| FEROCE72 | 2.044 | 2.024 | 2.015 | 2.019 | 2.039 | 2.015 | 2.019 | 2.039 | 2.044 | 2.024 | 2.028 | 3.284 | 1.642 | 1.642 | 34.859 | II |
| FEROCE73 | 2.036 | 2.026 | 2.019 | 2.029 | 2.028 | 2.019 | 2.029 | 2.028 | 2.036 | 2.026 | 2.028 | 3.291 | 1.646 | 1.646 | 35.373 | II |
| FEROCE74 | 2.044 | 2.031 | 2.024 | 2.03 | 2.037 | 2.024 | 2.03 | 2.037 | 2.044 | 2.031 | 2.033 | 3.298 | 1.649 | 1.649 | 35.724 | II |
| FEROCE75 | 2.051 | 2.02 | 2.025 | 2.025 | 2.045 | 2.025 | 2.025 | 2.045 | 2.051 | 2.02 | 2.033 | 3.287 | 1.644 | 1.644 | 34.980 | II |
| FEROCE76 | 2.044 | 2.026 | 2.02 | 2.028 | 2.048 | 2.02 | 2.028 | 2.048 | 2.044 | 2.026 | 2.033 | 3.285 | 1.642 | 1.642 | 35.101 | II |
| FEROCE77 | 2.053 | 2.03 | 2.026 | 2.028 | 2.052 | 2.026 | 2.028 | 2.052 | 2.053 | 2.030 | 2.038 | 3.289 | 1.645 | 1.645 | 35.651 | II |
| FEROCE78 | 2.05 | 2.026 | 2.023 | 2.026 | 2.037 | 2.026 | 2.037 | 2.05 | 2.026 | 2.023 | 2.032 | 3.274 | 1.637 | 1.637 | 35.912 | II |
| FEROCE79 | 2.057 | 2.026 | 2.015 | 2.033 | 2.059 | 2.033 | 2.059 | 2.057 | 2.026 | 2.015 | 2.038 | 3.257 | 1.629 | 1.629 | 34.954 | II |
| FEROCE80 | 2.038 | 2.045 | 2.028 | 2.02 | 2.027 | 2.028 | 2.045 | 2.038 | 2.027 | 2.02 | 2.032 | 3.269 | 1.635 | 1.635 | 35.770 | II |
| FEROCE81 | 2.043 | 2.045 | 2.029 | 2.021 | 2.021 | 2.029 | 2.045 | 2.043 | 2.021 | 2.021 | 2.032 | 3.268 | 1.634 | 1.634 | 35.826 | II |
| FEROCE82 | 2.043 | 2.014 | 2.009 | 2.027 | 2.027 | 2.027 | 2.027 | 2.043 | 2.014 | 2.009 | 2.024 | 3.249 | 1.625 | 1.625 | 35.719 | II |
| FEROCE83 | 2.046 | 2.014 | 2.012 | 2.023 | 2.038 | 2.023 | 2.038 | 2.046 | 2.014 | 2.012 | 2.027 | 3.254 | 1.627 | 1.627 | 35.783 | II |
| FEROCE84 | 2.04 | 2.007 | 2.006 | 2.022 | 2.038 | 2.022 | 2.038 | 2.04 | 2.007 | 2.006 | 2.023 | 3.250 | 1.625 | 1.625 | 35.797 | II |
| FEROCE85 | 2.063 | 2.026 | 1.948 | 2.04 | 2.059 | 1.948 | 2.026 | 2.063 | 2.059 | 2.04 | 2.027 | 3.286 | 1.643 | 1.643 | 35.437 | II |
| FERRIC01 | 2.06 | 2.06 | 2.063 | 2.06 | 2.063 | 2.064 | 2.07 | 2.064 | 2.081 | 2.081 | 2.067 | 3.395 | 1.701 | 1.695 | 0.507 | III |
| FUZGOY | 2.086 | 2.075 | 2.054 | 2.097 | 2.074 | 2.055 | 2.064 | 2.068 | 2.063 | 2.071 | 2.071 | 3.390 | 1.695 | 1.695 | 25.674 | III |
| GOFLUI | 2.093 | 1.95 | 1.98 | 1.975 | 1.941 | 1.955 | 2.035 | 2.001 | 2.028 | 1.94 | 1.999 | 3.135 | 1.564 | 1.573 | 4.696 | II |
| GOKRIH | 2.057 | 2.062 | 2.116 | 2.047 | 2.05 | 2.05 | 2.047 | 2.116 | 2.062 | 2.057 | 2.066 | 3.365 | 1.683 | 1.683 | 7.076 | III |
| GUNGAX | 2.068 | 2.064 | 2.075 | 2.094 | 2.08 | 2.08 | 2.072 | 2.033 | 2.023 | 2.074 | 2.066 | 3.403 | 1.708 | 1.696 | 4.352 | III |
| GUNGEB | 2.067 | 2.068 | 2.056 | 2.087 | 2.067 | 2.006 | 2.035 | 2.054 | 2.086 | 2.042 | 2.057 | 3.395 | 1.700 | 1.696 | 22.686 | III |
| GUNGIF | 2.074 | 2.064 | 2.057 | 2.037 | 2.05 | 2.057 | 2.037 | 2.05 | 2.074 | 2.064 | 2.056 | 3.397 | 1.699 | 1.699 | 32.324 | III |
| HARGIQ | 2.018 | 2.027 | 2.037 | 2.043 | 2.022 | 2.034 | 2.024 | 2.027 | 2.029 | 2.024 | 2.028 | 3.289 | 1.645 | 1.644 | 4.206 | II |
| HIGHUA | 2.082 | 2.092 | 2.079 | 2.08 | 2.069 | 2.08 | 2.079 | 2.092 | 2.082 | 2.069 | 2.080 | 3.404 | 1.702 | 1.702 | 0.333 | III |
| HUZLAR | 2.055 | 2.06 | 2.054 | 2.038 | 2.042 | 2.048 | 2.054 | 2.023 | 2.054 | 2.01 | 2.044 | 3.288 | 1.658 | 1.631 | 6.107 | II |
| IMUBEZ | 1.999 | 2.005 | 2.008 | 1.994 | 2.011 | 1.999 | 2.011 | 1.994 | 2.008 | 2.005 | 2.003 | 3.291 | 1.645 | 1.645 | 13.012 | II |
| INIKIZ (Fe1) | 2.069 | 2.05 | 2.085 | 2.074 | 2.029 | 2.074 | 2.029 | 2.069 | 2.05 | 2.085 | 2.061 | 3.405 | 1.703 | 1.703 | 35.837 | III |
| INIKIZ (Fe2) | 2.041 | 2.028 | 2.016 | 2.027 | 2.073 | 2.04 | 2.052 | 2.049 | 2.03 | 1.984 | 2.034 | 3.323 | 1.661 | 1.662 | 24.793 | II |
| INIKIZ (Fe3) | 2.085 | 2.066 | 2.078 | 2.077 | 2.083 | 2.078 | 2.077 | 2.083 | 2.085 | 2.066 | 2.078 | 3.387 | 1.693 | 1.693 | 35.733 | III |
| INIKIZ (Fe4) | 2.077 | 2.076 | 2.092 | 2.051 | 2.046 | 2.097 | 2.09 | 2.056 | 2.107 | 2.058 | 2.075 | 3.427 | 1.708 | 1.720 | 9.735 | III |
| INIKIZ (Fe5) | 2.007 | 2.027 | 2.036 | 2.035 | 1.99 | 2.036 | 2.027 | 2.007 | 1.99 | 2.035 | 2.019 | 3.262 | 1.631 | 1.631 | 35.683 | II |
| INIKIZ(Fe6) | 2.098 | 2.095 | 2.086 | 2.054 | 2.057 | 2.089 | 2.081 | 2.049 | 2.056 | 2.091 | 2.076 | 3.392 | 1.703 | 1.690 | 28.649 | III |
| IVUHIQ (Fe1) | 2.031 | 2.042 | 2.034 | 2.026 | 2.024 | 2.04 | 2.041 | 2.039 | 2.035 | 2.037 | 2.035 | 3.288 | 1.641 | 1.648 | 9.552 | II |
| IVUHIQ (Fe2) | 2.014 | 2.028 | 2.028 | 2.022 | 2.029 | 2.037 | 2.035 | 2.019 | 2.03 | 2.038 | 2.028 | 3.284 | 1.638 | 1.646 | 12.375 | II |
| JAHQAK | 2.038 | 2.057 | 2.069 | 2.066 | 2.051 | 2.058 | 2.078 | 2.06 | 2.046 | 2.048 | 2.057 | 3.407 | 1.705 | 1.702 | 16.247 | III |
| JALWIC (Fe1) | 2.051 | 2.042 | 2.065 | 2.077 | 2.042 | 2.113 | 2.072 | 2.018 | 2.078 | 2.07 | 2.063 | 3.400 | 1.707 | 1.694 | 4.775 | III |
| JALWIC (Fe2) | 2.095 | 2.073 | 2.044 | 2.081 | 2.082 | 2.07 | 2.071 | 2.073 | 2.085 | 2.069 | 2.074 | 3.410 | 1.706 | 1.704 | 2.117 | III |
| JUXZEJ | 2.074 | 2.065 | 2.011 | 2.024 | 2.076 | 2.026 | 2.043 | 2.05 | 2.056 | 2.07 | 2.049 | 3.39 | 1.693 | 1.698 | 1.978 | III |
| KAFMAG | 2.039 | 2.013 | 2.015 | 2.045 | 2.063 | 2.071 | 2.048 | 2.046 | 2.027 | 2.054 | 2.042 | 3.287 | 1.637 | 1.651 | 11.147 | II |
| KALGEL | 2.045 | 2.033 | 2.000 | 2.063 | 2.032 | 2.063 | 2.032 | 2.045 | 2.033 | 2.000 | 2.035 | 3.327 | 1.663 | 1.663 | 0.239 | II |
| KEFXUO (Fe1) | 2.06 | 2.079 | 2.045 | 2.038 | 2.032 | 2.038 | 2.032 | 2.06 | 2.079 | 2.045 | 2.051 | 3.319 | 1.660 | 1.660 | 35.92 | II |
| KEFXUO (Fe3) | 2.053 | 2.034 | 2.091 | 2.013 | 2.039 | 2.013 | 2.039 | 2.053 | 2.034 | 2.091 | 2.046 | 3.397 | 1.698 | 1.698 | 32.032 | III |
| KEFXUO03 (Fe1) | 2.086 | 2.101 | 2.095 | 2.074 | 2.068 | 2.074 | 2.068 | 2.086 | 2.101 | 2.095 | 2.085 | 3.399 | 1.699 | 1.699 | 36.000 | III |
| KEFXUO03 (Fe2) | 2.09 | 2.064 | 2.042 | 2.055 | 2.086 | 2.055 | 2.086 | 2.09 | 2.064 | 2.042 | 2.067 | 3.356 | 1.678 | 1.678 | 35.987 | III |
| KOVMUD | 2.046 | 2.037 | 2.063 | 2.063 | 2.037 | 2.061 | 2.051 | 2.051 | 2.061 | 2.052 | 2.052 | 3.366 | 1.694 | 1.672 | 0.069 | III |
| KUVNOE | 2.046 | 2.041 | 2.041 | 2.044 | 2.047 | 2.049 | 2.039 | 2.051 | 2.058 | 2.051 | 2.047 | 3.299 | 1.648 | 1.651 | 2.706 | II |
| KUVNOE01 | 2.049 | 2.029 | 2.04 | 2.044 | 2.048 | 2.053 | 2.039 | 2.038 | 2.043 | 2.052 | 2.043 | 3.301 | 1.649 | 1.652 | 1.307 | II |
| LAWTIM | 2.093 | 2.092 | 2.092 | 2.11 | 2.092 | 2.11 | 2.092 | 2.093 | 2.092 | 2.092 | 2.096 | 3.425 | 1.713 | 1.713 | 33.351 | III |
| LETSAF | 2.082 | 2.077 | 2.065 | 2.067 | 2.076 | 2.07 | 2.053 | 2.071 | 2.072 | 2.08 | 2.071 | 3.402 | 1.700 | 1.702 | 4.472 | III |
| LETSAF01 (Fe1) | 2.114 | 2.091 | 2.116 | 2.091 | 2.073 | 2.06 | 2.08 | 2.095 | 2.091 | 2.095 | 2.091 | 3.422 | 1.715 | 1.707 | 3.588 | III |
| LETSAF01 (Fe2) | 2.068 | 2.098 | 2.116 | 2.059 | 2.073 | 2.097 | 2.093 | 2.078 | 2.08 | 2.117 | 2.088 | 3.407 | 1.702 | 1.705 | 7.904 | III |
| LIYNAI (Fe1) | 2.076 | 2.062 | 2.067 | 2.072 | 2.022 | 2.062 | 2.072 | 2.081 | 2.056 | 2.055 | 2.062 | 3.397 | 1.699 | 1.701 | 21.828 | III |
| LIYNAI (Fe2) | 2.062 | 2.054 | 2.078 | 2.083 | 2.057 | 2.074 | 2.095 | 2.09 | 2.073 | 2.061 | 2.073 | 3.401 | 1.697 | 1.705 | 3.528 | III |
| LONGOK | 2.039 | 2.038 | 2.044 | 2.046 | 2.044 | 2.039 | 2.045 | 2.05 | 2.037 | 2.041 | 2.042 | 3.293 | 1.646 | 1.647 | 1.214 | II |
| LUZJIB | 2.024 | 2.03 | 2.015 | 2.024 | 2.024 | 2.017 | 2.027 | 2.032 | 2.053 | 2.022 | 2.027 | 3.288 | 1.646 | 1.643 | 4.092 | II |
| MACWUJ | 2.046 | 2.1 | 2.115 | 2.088 | 2.045 | 2.057 | 2.086 | 2.097 | 2.077 | 2.057 | 2.077 | 3.412 | 1.708 | 1.705 | 8.353 | III |
| NAHMEP | 2.036 | 2.04 | 2.035 | 2.031 | 2.035 | 2.035 | 2.031 | 2.035 | 2.036 | 2.04 | 2.035 | 3.292 | 1.646 | 1.646 | 35.310 | II |
| NIRSAK | 2.082 | 2.085 | 2.087 | 2.064 | 2.08 | 2.074 | 2.109 | 2.109 | 2.083 | 2.06 | 2.083 | 3.415 | 1.711 | 1.704 | 19.850 | III |
| NOHKAX | 2.054 | 2.032 | 2.037 | 2.034 | 2.047 | 2.034 | 2.047 | 2.054 | 2.032 | 2.037 | 2.041 | 3.309 | 1.655 | 1.655 | 35.656 | II |
| NUMXAU | 2.068 | 2.068 | 2.093 | 2.09 | 2.093 | 2.047 | 2.045 | 2.047 | 2.048 | 2.048 | 2.065 | 3.402 | 1.710 | 1.692 | 0.141 | III |
| OCUJIF | 2.02 | 2.006 | 1.996 | 1.974 | 1.996 | 1.974 | 1.996 | 2.02 | 2.006 | 1.996 | 1.998 | 3.279 | 1.639 | 1.639 | 33.580 | II |
| POTTOH (Fe1) | 2.079 | 2.093 | 2.087 | 2.08 | 2.073 | 2.083 | 2.091 | 2.091 | 2.085 | 2.074 | 2.084 | 3.41 | 1.705 | 1.705 | 4.606 | III |
| POTTOH (Fe2) | 2.039 | 2.036 | 2.035 | 2.035 | 2.033 | 2.035 | 2.035 | 2.042 | 2.041 | 2.031 | 2.036 | 3.282 | 1.640 | 1.642 | 0.702 | II |
| POTTOH (Fe3) | 2.079 | 2.076 | 2.082 | 2.08 | 2.087 | 2.08 | 2.071 | 2.07 | 2.081 | 2.083 | 2.079 | 3.401 | 1.702 | 1.699 | 4.256 | III |
| PUVFIV | 2.06 | 2.06 | 2.061 | 2.067 | 2.061 | 2.064 | 2.069 | 2.064 | 2.08 | 2.08 | 2.067 | 3.393 | 1.698 | 1.695 | 0.166 | III |
| QIDREB | 2.044 | 2.037 | 2.026 | 2.032 | 2.039 | 2.043 | 2.044 | 2.043 | 2.045 | 2.042 | 2.039 | 3.290 | 1.637 | 1.653 | 8.297 | II |
| RAMTII (Fe1) | 2.053 | 2.075 | 2.108 | 2.105 | 2.071 | 2.108 | 2.105 | 2.071 | 2.053 | 2.075 | 2.082 | 3.393 | 1.697 | 1.697 | 35.970 | III |
| RAMTII (Fe2) | 2.06 | 2.071 | 2.09 | 2.091 | 2.072 | 2.09 | 2.071 | 2.06 | 2.072 | 2.091 | 2.077 | 3.379 | 1.690 | 1.690 | 35.978 | III |
| RETMOS (Fe1) | 2.101 | 2.111 | 2.071 | 2.091 | 2.119 | 2.091 | 2.119 | 2.101 | 2.111 | 2.071 | 2.099 | 3.432 | 1.716 | 1.716 | 35.505 | III |
| RETMOS (Fe2) | 2.069 | 2.096 | 2.035 | 2.053 | 2.082 | 2.087 | 2.074 | 2.076 | 2.063 | 2.083 | 2.072 | 3.397 | 1.704 | 1.693 | 19.144 | III |
| TIBCUE | 1.872 | 2.019 | 2.044 | 2.087 | 2.028 | 2.044 | 2.087 | 2.028 | 1.872 | 2.019 | 2.010 | 3.205 | 1.602 | 1.602 | 32.946 | II |
| VOVLOJ | 2.04 | 2.042 | 2.045 | 2.035 | 2.03 | 2.034 | 2.041 | 2.045 | 2.049 | 2.032 | 2.039 | 3.294 | 1.647 | 1.647 | 0.174 | II |
| XITDIP | 2.109 | 2.089 | 2.081 | 2.088 | 2.08 | 2.079 | 2.088 | 2.091 | 2.102 | 2.069 | 2.088 | 3.417 | 1.711 | 1.707 | 25.129 | III |
| YIPQIX | 2.06 | 2.06 | 2.066 | 2.059 | 2.066 | 2.065 | 2.071 | 2.065 | 2.078 | 2.078 | 2.067 | 3.380 | 1.692 | 1.689 | 0.308 | III |
| ZAGXEK | 2.087 | 2.062 | 2.066 | 2.064 | 2.046 | 2.055 | 2.063 | 2.058 | 2.055 | 2.024 | 2.058 | 3.415 | 1.709 | 1.708 | 18.764 | III |
| ZOZLEF | 2.097 | 2.08 | 2.067 | 2.076 | 2.094 | 2.098 | 2.085 | 2.073 | 2.078 | 2.099 | 2.085 | 3.412 | 1.707 | 1.706 | 0.892 | III |
References
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin, Germany, 1983. [Google Scholar]
- Nishimoto, Y.; Yokogawa, D.; Yoshikawa, H.; Awaga, K.; Irle, S. Super-Reduced Polyoxometalates: Excellent Molecular Cluster Battery Components and Semipermeable Molecular Capacitors. J. Am. Chem. Soc. 2010, 136, 9042–9052. [Google Scholar] [CrossRef] [PubMed]
- Ouahab, L.; Bencharif, M.; Grandjean, D. Premiers solides où coexistent des états de valence mixte sur les entités organiques et minérales: Préparation et données structurales du ternaire (TTF)6PWI2O40 (Et4N)2. C. R. Acad. Sci. Paris Sér. II 1988, 307, 749–752. [Google Scholar]
- Ouahab, L.; Bencharif, M.; Mhanni, A.; Pelloquin, D.; Halet, J.F.; Peña, O.; Padiou, J.; Grandjean, D.; Garrigou Lagrange, C.; Amiell, J.; et al. Preparations, x-ray crystal structures, EH band calculations and physical properties of [(TTF)6(H)(XM12O40)(Et4N)] (M = tungsten, molybdenum; X = phosphorus, silicon): Evidence of electron transfer between organic donors and polyoxometalates. Chem. Mater. 1992, 4, 666–674. [Google Scholar] [CrossRef]
- Gómez-García, C.J.; Borrás-Almenar, J.J.; Coronado, E.; Delhaès, P.; Garrigou-Lagrange, C.; Baker, L.C.W. Organic-inorganic salts made by TTF and magnetic clusters. Synth. Met. 1993, 56, 2023–2027. [Google Scholar] [CrossRef]
- Triki, S.; Ouahab, L.; Padiou, J.; Grandjean, D. The use of polyoxometallates as acceptors in charge transfer salts: Preparation, X-ray crystal structures and preliminary spectroscopic characterizations of D3M6O19, D = TTF, TMTSF; M = Mo, W. J. Chem. Soc. Chem. Commun. 1989, 15, 1068–1070. [Google Scholar] [CrossRef]
- Davidson, A.; Boubekeur, K.; Pénicaud, A.; Auban, P.; Lenoir, C.; Batail, P.; Hervé, G. Mixed-valence bis(ethylenedithio)tetrathiafulvalenium (BEDT-TTF) monolayers sandwiched between extended close-packed Keggin-type molecular metal oxide cluster arrays: Synthesis, unprecedented acentric structure and preliminary conducting and e.s.r. properties of (BEDT-TTF)8SiW12O40. J. Chem. Soc. Chem. Commun. 1989, 18, 1373–1374. [Google Scholar]
- Triki, S.; Ouahab, L.; Halet, J.F.; Peña, O.; Padiou, J.; Grandjean, D.; Garrigou-Lagrange, C.; Delhaès, P. Preparation and properties of tetrathia- and tetramethyltetraselena-fulvalene salts of [M6O19]2− (M = Mo or W). J. Chem. Soc. Dalton Trans. 1992, 7, 1217–1227. [Google Scholar] [CrossRef]
- Gómez-García, C.J.; Ouahab, L.; Gimenez-Saiz, C.; Triki, S.; Coronado, E.; Delhaès, P. Coexistence of Mobile and Localized Electrons in Bis(ethylene)dithiotetrathiafulvalene (BEDT-TTF) Radical Salts with Paramagnetic Polyoxometalates: Synthesis and Physical Properties of (BEDT-TTF)8[CoW12O40]·5.5 H2O. Angew. Chem. Int. Ed. Engl. 1994, 33, 223–226. [Google Scholar] [CrossRef]
- Ouahab, L. Coordination complexes in conducting and magnetic molecular materials. Coord. Chem. Rev. 1998, 178, 1501–1534. [Google Scholar] [CrossRef]
- Miller, J.S.; Calabrese, J.C.; Epstein, A.J.; Bigelow, R.W.; Zhang, J.H.; Reiff, W.M. Ferromagnetic properties of one-dimensional decamethylferrocenium tetracyanoethylenide (1:1): [Fe(η5-C5Me5)2]+[TCNE]−. J. Chem. Soc. Chem. Commun. 1986, 13, 1026–1028. [Google Scholar] [CrossRef]
- Miller, J.S.; Epstein, A.J.; Reiff, W.M. Linear Chain Ferromagnetic Compounds—Recent Progress. Mol. Cryst. Liq. Cryst. 1985, 120, 27–34. [Google Scholar] [CrossRef]
- Coronado, E.; Gomez-García, C.J. Polyoxometalate-Based Molecular Materials. Chem. Rev. 1998, 98, 273–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xue, G.; Hu, H.; Gao, Q.; Fu, F.; Wang, J. Synthesis and crystal structure of a new charge transfer salt [NBu4]6H[Fe(C5H5)2][PMoVMo11O40]2. J. Mol. Struct. 2006, 787, 101–105. [Google Scholar] [CrossRef]
- Li, Z.; Cui, R.; Xue, G.; Hu, H.; Fu, F.; Wang, J. Synthesis, structure and absorption spectrum of a new charge transfer salt [Fe(C5H5)2]4H[GeMo12O40]·CH3CN·H2O. J. Coord. Chem. 2009, 62, 1951–1958. [Google Scholar] [CrossRef]
- Li, Z.; Cui, R.; Liu, B.; Xue, G.; Hu, H.; Fu, F.; Wang, J. Structural and property characterization of two new charge-transfer salts based on Keggin ions and ferrocene. J. Mol. Struct. 2009, 920, 436–440. [Google Scholar] [CrossRef]
- Le Maguerès, P.; Ouahab, L.; Golhen, S.; Grandjean, D.; Peña, O.; Jegaden, J.C.; Gomez-Garcia, C.J.; Delhaès, P. Diamagnetic and Paramagnetic Keggin Polyoxometalate Salts Containing 1-D and 3-D decamethylferrocenium Networks: Preparation, Crystal Structures and Magnetic Properties of [Fe(C5Me5)2]4(POM)(solv)n (POM = [SiMo12O40]4−, [SiW12O40]4−, [PMo12O40]4−, [HFeW12O40]4−; solv = H2O, C3H7ON, CH3CN). Inorg. Chem. 1994, 33, 5180–5187. [Google Scholar]
- Golhen, S.; Ouahab, L.; Grandjean, D.; Molinié, P. Preparation, Crystal Structures and Magnetic and ESR Properties of Molecular Assemblies of Ferrocenium Derivatives and Paramagnetic Polyoxometalates. Inorg. Chem. 1998, 37, 1499–1506. [Google Scholar] [CrossRef]
- Juraja, S.; Vu, T.; Richardt, P.J.S.; Bond, A.M.; Cardwell, T.J.; Cashion, J.D.; Fallon, G.D.; Lazarev, G.; Moubaraki, B.; Murray, K.S.; et al. Electrochemical, Spectroscopic, Structural and Magnetic Characterization of the Reduced and Protonated α-Dawson Anions in [Fe(η5-C5Me5)2]5[HS2Mo18O62]·3HCONMe2·2Et2O and [NBu4]5[HS2Mo18O62]·2H2O. Inorg. Chem. 2002, 41, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Veya, P.L.; Kochi, J.K. Structural and spectral characterization of novel charge-transfer salts of polyoxometalates and the cationic ferrocenyl donor. J. Organomet. Chem. 2002, 41, 1072–1078. [Google Scholar] [CrossRef]
- Xiong, J.; Niu, Y.; Xu, H.; Cao, G.; Liu, B.; Hu, H.; Xue, G. Charge-transfer salts based on Lindqvist and Keggin polyoxoanion acceptors and ferrocenyl cationic donors. New J. Chem. 2012, 36, 1224–1230. [Google Scholar] [CrossRef]
- Li, Z.; Liu, B.; Xu, H.; Xue, G.; Hu, H.; Fu, F.; Wang, J. Preparation, crystal structures, EPR and reflectance spectra of two new charge-transfer salts, [CpFeCpCH2N(CH3)3]4[XMo12O40]·nCH3CN (n = 0 for X = P or n = 1 for X = Ge). J. Organomet. Chem. 2009, 694, 2210–2216. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Liu, B.; Xue, G.; Hu, H.; Fu, F.; Wang, J. Charge-Transfer Salts via Cocrystallization of the Cationic Ferrocenyl Donor with Polyoxometalate Acceptors. Cryst. Growth Des. 2010, 10, 1096–1103. [Google Scholar] [CrossRef]
- Stark, J.L.; Young, V.G., Jr.; Maatta, E.A. A Functionalized Polyoxometalate Bearing a Ferrocenylimido Ligand: Preparation and Structure of [(FcN)Mo6O18]2−. Angew. Chem., Int. Ed. Engl. 1995, 34, 2547–2548. [Google Scholar] [CrossRef]
- Kang, J.; Nelson, J.A.; Lu, M.; Xie, B.; Peng, Z.; Powell, D.R. Charge-Transfer Hybrids Containing Covalently Bonded Polyoxometalates and Ferrocenyl Units. Inorg. Chem. 2004, 43, 6408–6413. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.; Gyepes, R.; Císařová, I.; Štěpnička, P. Synthesis, structural characterisation and bonding in an anionic hexavanadate bearing redox-active ferrocenyl groups at the periphery. New. J. Chem. 2010, 34, 2749–2756. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- San Felices, L.; Vitoria, P.; Gutiérrez-Zorrilla, J.M.; Lezama, L.; Reinoso, S. Hybrid Inorganic−Metalorganic Compounds Containing Copper (II)-Monosubstituted Keggin Polyanions and Polymeric Copper (I) Complexes. Inorg. Chem. 2006, 45, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, N.; Ganesan, A.; Chantler, C.T.; Wang, F. Differentiation of ferrocene D5d and D5h conformers using IR spectroscopy. J. Organomet. Chem. 2012, 713, 51–59. [Google Scholar] [CrossRef]
- Reinoso, S.; Vitoria, P.; San Felices, L.; Lezama, L.; Gutiérrez-Zorrilla, J.M. Organic–Inorganic Hybrids Based on Novel Bimolecular [Si2W22Cu2O78(H2O)]12− Polyoxometalates and the Polynuclear Complex Cations [Cu(ac)(phen)(H2O)]nn+ (n = 2, 3). Chem. Eur. J. 2005, 11, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.D.; Alternatt, D. Bond-Valence Parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Duggan, D.M.; Hendrickson, D.N. Electronic structure of various ferricenium systems as inferred from Raman, infrared, low-temperature electronic absorption and electron paramagnetic resonance measurements. Inorg. Chem. 1975, 14, 955–970. [Google Scholar] [CrossRef]
- Connelly, N.G.; Geiger, W.E. Chemical Redox Agents for Organometallic Chemistry. Chem. Rev. 1996, 96, 877–910. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Bhavya Deepthi, S.; Giribabu, L.; Sridhar, B.; Sujitha, P.; Kumar, C.G.; Ramakrishna, K.V.S. Synthesis, Crystal Structure, Electronic Spectroscopy, Electrochemistry and Biological Studies of Ferrocene–Carbohydrate Conjugates. Eur. J. Inorg. Chem. 2012, 13, 2267–2277. [Google Scholar] [CrossRef]
- Churchill, M.R.; Li, Y.J.; Nalewajek, D.; Schaber, P.M.; Dorfman, J. Preparation crystal and molecular structure and properties of tetrakis(ferrocenecarboxylato)bis(tetrahydrofuran)dicopper (II). A structure containing both eclipsed and staggered ferrocenyl fragments. Inorg. Chem. 1985, 24, 2684–2687. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Gibb, T.C. Mössbauer Spectroscopy; Chapman and Hall: London, UK, 1971. [Google Scholar]
- Ernst, R.D.; Wilson, D.R.; Herber, R.H. Bonding, Hyperfine Interactions and Lattice Dynamics of Bis(pentadienyl)iron Compounds. J. Am. Chem. Soc. 1984, 106, 1646–1650. [Google Scholar] [CrossRef]
- Magalhaes, C.I.R.; Gomes, A.C.; Lopes, A.D.; Gonçalves, I.S.; Pillinger, M.; Jin, E.; Kim, I.; Ko, Y.H.; Kim, K.; Nowik, I.; et al. Ferrocene and ferrocenium inclusion compounds with cucurbiturils: A study of metal atom dynamics probed by Mossbauer spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 21548–21555. [Google Scholar] [CrossRef] [PubMed]
- Tézé, A.; Hervé, G. Early transition metal polyoxoanions. Inorg. Synth. 1990, 27, 85–96. [Google Scholar]
- Brand, R.A. Normos Mössbauer Fitting Program; Universität Dortmund: Dortmund, Germany, 2007. [Google Scholar]
- CrysAlis Pro CCD V38.2 and RED; Oxford Diffraction, Ltd.: Oxford, UK, 2009.
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, T. SIR2004: An Improved Tool for Crystal Structure Determination and Refinement. J. Appl. Crystallogr. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |








| Fe1 | Fe2 | Fe3 | |||
|---|---|---|---|---|---|
| C1 | 2.084 (9) | C11 | 2.044 (9) | C21A | 2.077 (18) |
| C2 | 2.086 (8) | C12 | 2.059 (12) | C22A | 2.048 (19) |
| C3 | 2.080 (7) | C13 | 2.057 (11) | C23A | 2.02 (2) |
| C4 | 2.075 (7) | C14 | 2.043 (10) | C24A | 2.037 (10) |
| C5 | 2.078 (8) | C15 | 2.034 (10) | C25A | 2.019 (10) |
| C6 | 2.060 (9) | C16 | 2.062 (9) | C21B | 1.98 (3) |
| C7 | 2.073 (8) | C17 | 2.084 (8) | C22B | 2.03 (2) |
| C8 | 2.089 (10) | C18 | 2.083 (8) | C23B | 2.09 (2) |
| C9 | 2.086 (10) | C19 | 2.062 (8) | C24B | 2.095 (19) |
| C10 | 2.068 (9) | C20 | 2.049 (9) | C25B | 2.03 (2) |
| Average | 2.08 | Average | 2.06 | Average | 2.05 |
| Fe···Cg(Cp1) | 1.704 | Fe···Cg(Cp3) | 1.701 | Fe···Cg(Cp5A) | 1.648 |
| Fe···Cg(Cp2) | 1.699 | Fe···Cg(Cp4) | 1.708 | Fe···Cg(Cp5B) | 1.667 |
| Torsion angle | 0.94 | 34.53 | - | ||
| Symmetry | D5h | D5d | Disordered |
| π-π Interactions | Cg(Cp)··· Plane | ANG | Cg(Cp)··· Cg(Cp) | Slippage | |
|---|---|---|---|---|---|
| Stacking | Cp1-Cp1i | 3.445 | 0.00 | 3.497 | 0.603 |
| Cp2-Cp2ii | 3.326 | 0.00 | 3.829 | 1.898 | |
| T-type | Cp1-Cp3iii | 3.728 | 89.4 (5) | 5.145 | 3.545 |
| Cp1-Cp5A | 4.264 | 88.7 (9) | 4.537 | 1.550 | |
| Cp1-Cp5B | 4.244 | 87.1 (11) | 4.480 | 1.435 | |
| Cp2-Cp4 | 4.680 | 88.3 (5) | 4.720 | 0.618 | |
| Cp3-Cp5Aiv | 4.938 | 88.4 (10) | 5.079 | 1.188 | |
| Cp3-Cp5Biv | 4.873 | 87.4 (11) | 4.989 | 1.061 | |
| D-A | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|
| O1M–H1M···O13 | 0.84 | 2.25 | 3.018 (12) | 152 |
| C1M–H1M1···O14 | 0.98 | 2.36 | 3.281 (15) | 157 |
| C1M–H1M1···O4i | 0.98 | 2.88 | 3.168 (16) | 98 |
| C4-H4···O4i | 0.95 | 2.44 | 3.182 (12) | 135 |
| C7-H7···O5ii | 0.95 | 2.55 | 3.450 (12) | 157 |
| C8-H8···O6i | 0.95 | 2.48 | 3.286 (10) | 143 |
| C11-H11···O12iii | 0.95 | 2.56 | 3.389 (13) | 145 |
| C12-H12···O2iii | 0.95 | 2.48 | 3.225 (14) | 135 |
| C13-H13···O3iv | 0.95 | 2.53 | 3.342 (14) | 144 |
| C13-H13···O8iv | 0.95 | 2.51 | 3.371 (15) | 150 |
| C14-H14···O1 | 0.95 | 2.46 | 3.380 (14) | 164 |
| C15-H15···O6v | 0.95 | 2.42 | 3.352 (16) | 167 |
| C16-H16···O6iii | 0.95 | 2.44 | 3.240 (11) | 142 |
| C18-H18···O7iv | 0.95 | 2.59 | 3.414 (15) | 146 |
| C19-H19···O1iv | 0.95 | 2.59 | 3.308 (11) | 133 |
| C20-H20···O15 | 0.95 | 2.56 | 3.272 (11) | 132 |
| C24B-H24B···O16iiii | 0.95 | 2.59 | 3.427 (12) | 147 |
| Signal | δ (mm/s) | Multiplicity | Δ (mm/s) | Oxidation State | Area | Atomic % |
|---|---|---|---|---|---|---|
| Fe1 | 0.27 | 1 | - | III | 2 | 40 |
| Fe2 | 0.44 | 1 | - | III | 2 | 40 |
| Fe3 | 0.71 | 2 | 1.17 | II | 1 | 20 |
| Parameters | 1 |
|---|---|
| Formula | C52H58Fe5O42SiW12 |
| FW (g mol−1) | 3868.40 |
| Crystal System | Triclinic |
| Space Group | P–1 |
| a (Å) | 12.5120 (5) |
| b(Å) | 13.0831 (6) |
| c (Å) | 13.3076 (6) |
| α (°) | 117.296 (5) |
| β (°) | 95.632 (3) |
| γ (°) | 101.909 (4) |
| V (Å3) | 1874.03 (18) |
| Z | 1 |
| ρcalcd (g cm−3) | 3.478 |
| μ (mm−1) | 19.651 |
| Collected Reflections | 19620 |
| Unique Reflections (Rint) | 8904 (0.036) |
| Observed Reflections [I > 2σ(I)] | 6061 |
| Parameters | 505 |
| Restrains | 120 |
| R(F) a [I > 2σ(I)] | 0.036 |
| wR(F2) a [all data] | 0.072 |
| GoF | 0.860 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artetxe, B.; Iturrospe, A.; Vitoria, P.; Ruiz-Bilbao, E.; S. Garitaonandia, J.; Gutiérrez-Zorrilla, J.M. A Charge-Transfer Salt Based on Ferrocene/Ferrocenium Pairs and Keggin-Type Polyoxometalates. Molecules 2018, 23, 3150. https://doi.org/10.3390/molecules23123150
Artetxe B, Iturrospe A, Vitoria P, Ruiz-Bilbao E, S. Garitaonandia J, Gutiérrez-Zorrilla JM. A Charge-Transfer Salt Based on Ferrocene/Ferrocenium Pairs and Keggin-Type Polyoxometalates. Molecules. 2018; 23(12):3150. https://doi.org/10.3390/molecules23123150
Chicago/Turabian StyleArtetxe, Beñat, Amaia Iturrospe, Pablo Vitoria, Estibaliz Ruiz-Bilbao, José S. Garitaonandia, and Juan M. Gutiérrez-Zorrilla. 2018. "A Charge-Transfer Salt Based on Ferrocene/Ferrocenium Pairs and Keggin-Type Polyoxometalates" Molecules 23, no. 12: 3150. https://doi.org/10.3390/molecules23123150