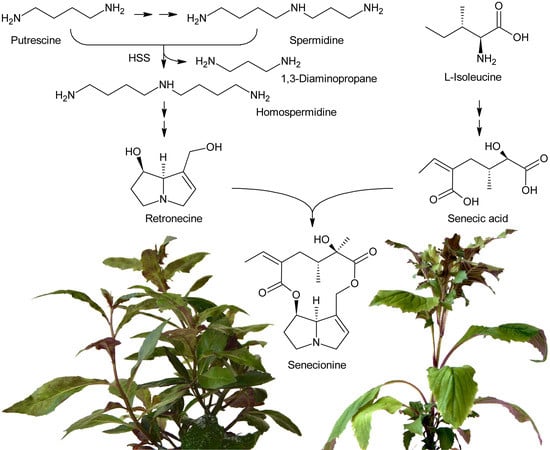
Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants
Abstract
:1. Introduction
2. Structural Diversity of Pyrrolizidine Alkaloids
2.1. Diversity of Necine Bases
2.2. Diversity of Necic Acids
2.3. Linkage Patterns of Necine Bases with Necic Acids
2.4. Modification and Conjugation of Pyrrolizidine Alkaloids
3. Biosynthesis of Pyrrolizidine Alkaloids
3.1. Biosynthesis of Necine Bases
3.1.1. Homospermidine Synthase
3.1.2. Copper-Dependent Diamine Oxidases and Cyclization of the Dialdehyde
3.1.3. Further Downstream Reactions
3.2. Biosynthesis of Necic Acids
3.2.1. Tiglic Acid and Related C5 Necic Acids
3.2.2. C7 Necic Acids
3.2.3. Monocrotalic Acid and Related Compounds
3.2.4. Senecic Acid and Senecic Acid-Derived Compounds
4. Regulation of Pyrrolizidine Alkaloid Levels and Biosynthesis
5. Biological Activity
5.1. Role in Plant Ecology
5.2. Toxicity of Pyrrolizidine Alkaloids and Mechanisms for their Detoxification
5.3. Beneficial Properties of Certain PAs
6. Occurrence of Pyrrolizidine Alkaloids in Crop Plants
6.1. Borago officinalis
6.2. Crassocephalum crepidioides
6.3. Gynura
6.4. Lolium
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Macel, M. Attract and deter: A dual role for pyrrolizidine alkaloids in plant-insect interactions. Phytochem. Rev. 2011, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ober, D.; Hartmann, T. Homospermidine synthase, the first pathway-specific enzyme of pyrrolizidine alkaloid biosynthesis, evolved from deoxyhypusine synthase. Proc. Natl. Acad. Sci. USA 1999, 96, 14777–14782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA. Scientific opinion on pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 2406. [Google Scholar] [CrossRef]
- Molyneux, R.J.; Gardner, D.L.; Colegate, S.M.; Edgar, J.A. Pyrrolizidine alkaloid toxicity in livestock: A paradigm for human poisoning? Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Grossman, R.B.; Nagabhyru, P.; Faulkner, J.R.; Mallik, U.P. Loline alkaloids: Currencies of mutualism. Phytochemistry 2007, 68, 980–996. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Bhardwaj, M.; Faulkner, J.R.; Nagabhyru, P.; Charlton, N.D.; Higashi, R.M.; Miller, A.F.; Young, C.A.; Grossman, R.B.; Schardl, C.L. Ether bridge formation in loline alkaloid biosynthesis. Phytochemistry 2014, 98, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Bhardwaj, M.; Nagabhyru, P.; Grossman, R.B.; Schardl, C.L. Enzymes from fungal and plant origin required for chemical diversification of insecticidal loline alkaloids in grass-Epichloe symbiota. PLoS ONE 2014, 9, e115590. [Google Scholar] [CrossRef]
- Bunsupa, S.; Yamazaki, M.; Saito, K. Quinolizidine alkaloid biosynthesis: Recent advances and future prospects. Front. Plant Sci. 2012, 3, 239. [Google Scholar] [CrossRef]
- Kim, N.; Estrada, O.; Chavez, B.; Stewart, C.; D’Auria, J.C. Tropane and Granatane Alkaloid Biosynthesis: A Systematic Analysis. Molecules 2016, 21, 1510. [Google Scholar] [CrossRef]
- Moreira, R.; Pereira, D.M.; Valentao, P.; Andrade, P.B. Pyrrolizidine Alkaloids: Chemistry, Pharmacology, Toxicology and Food Safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef]
- Robertson, J.; Stevens, K. Pyrrolizidine alkaloids: Occurrence, biology, and chemical synthesis. Nat. Prod. Rep. 2017, 34, 62–89. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Nonaka, H.; Furumai, T.; Igarashi, Y. Cremastrine, a pyrrolizidine alkaloid from Cremastra appendiculata. J. Nat. Prod. 2005, 68, 572–573. [Google Scholar] [CrossRef] [PubMed]
- Lindström, B.; Lüning, B. Studies on Orchidaceae alkaloids XIII. A new alkaloid, laburnine acetate, from Vanda cristata Lindl. Acta Chem. Scand. 1969, 23, 3352–3354. [Google Scholar] [CrossRef]
- Hoang le, S.; Tran, M.H.; Lee, J.S.; To, D.C.; Nguyen, V.T.; Kim, J.A.; Lee, J.H.; Woo, M.H.; Min, B.S. Anti-inflammatory Activity of Pyrrolizidine Alkaloids from the Leaves of Madhuca pasquieri (Dubard). Chem. Pharm. Bull. 2015, 63, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Freer, A.A.; Kelly, H.A.; Robins, D.J. Rosmarinine: A pyrrolizidine alkaloid. Acta Cryst. C 1986, 42, 1348–1350. [Google Scholar] [CrossRef]
- Roeder, E.; Wiedenfeld, H. Pyrrolizidine alkaloids in medicinal plants of Mongolia, Nepal and Tibet. Pharmazie 2009, 64, 699–716. [Google Scholar] [CrossRef]
- Roeder, E. Medicinal plants in Europe containing pyrrolizidine alkaloids. Pharmazie 1995, 50, 83–98. [Google Scholar] [PubMed]
- Tasso, B.; Novelli, F.; Sparatore, F.; Fasoli, F.; Gotti, C. (+)-Laburnamine, a natural selective ligand and partial agonist for the alpha4beta2 nicotinic receptor subtype. J. Nat. Prod. 2013, 76, 727–731. [Google Scholar] [CrossRef]
- Suri, O.P.; Jamwal, R.S.; Suri, K.A.; Atal, C.K. Ehretinine, a novel pyrrolizidine alkaloid from Ehretia aspera. Phytochemistry 1980, 19, 1273–1274. [Google Scholar] [CrossRef]
- Villanueva-Canongo, C.; Perez-Hernandez, N.; Hernandez-Carlos, B.; Cedillo-Portugal, E.; Joseph-Nathan, P.; Burgueno-Tapia, E. Complete 1H NMR assignments of pyrrolizidine alkaloids and a new eudesmanoid from Senecio polypodioides. Magn. Reson. Chem. 2014, 52, 251–257. [Google Scholar] [CrossRef]
- Alali, F.Q.; Tahboub, Y.R.; Ibrahim, E.S.; Qandil, A.M.; Tawaha, K.; Burgess, J.P.; Sy, A.; Nakanishi, Y.; Kroll, D.J.; Oberlies, N.H. Pyrrolizidine alkaloids from Echium glomeratum (Boraginaceae). Phytochemistry 2008, 69, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Roeder, E.; Wiedenfeld, H.; Jost, E.J. Tussilagine—A new pyrrolizidine alkaloid from Tussilago farfara. Planta Med. 1981, 43, 99–102. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, A.; Abdel-Ghani, A.; Wink, M. Pyrrolizidine alkaloids from Onosma arenaria (Boraginaceae). Biochem. Syst. Ecol. 2003, 31, 477–485. [Google Scholar] [CrossRef]
- Brandänge, S.; Granelli, I.; Johanson, R.; Hytta, R.; van der Hoeven, M.G.; Swahn, C.G. Studies on Orchidaceae Alkaloids. XXXVI. Alkaloids from some Vanda and Vandopsis Species. Acta Chem. Scand. 1973, 27, 1096–1097. [Google Scholar] [CrossRef]
- El-Shazly, A.; El-Domiaty, M.; Witte, L.; Wink, M. Pyrrolizidine alkaloids in members of the Boraginaceae from Sinai (Egypt). Biochem. Syst. Ecol. 1998, 26, 619–636. [Google Scholar] [CrossRef]
- Roeder, E.; Wiedenfeld, H.; Hoenig, A. Pyrrolizidinalkaloide aus Senecio aureus. Planta Med. 1983, 49, 57–59. [Google Scholar] [CrossRef]
- Hikichi, M.; Asada, Y.; Furuya, T. Ligularidine, a new pyrrolizidine alkaloid from Ligularia dentata. Tetrahedron Lett. 1979, 20, 1233–1236. [Google Scholar] [CrossRef]
- Toppel, G.; Witte, L.; Riebesehl, B.; Borstel, K.V.; Hartmann, T. Alkaloid patterns and biosynthetic capacity of root cultures from some pyrrolizidine alkaloid producing Senecio species. Plant Cell Rep. 1987, 6, 466–469. [Google Scholar] [CrossRef]
- Ravi, S.; Ravikumar, R.; Lakshmanan, A.J. Pyrrolizidine alkaloids from Cynoglossum furcatum. J. Asian Nat. Prod. Res. 2008, 10, 349–354. [Google Scholar] [CrossRef]
- Culvenor, C.C.; Edgar, J.A.; Smith, L.W. Pyrrolizidine alkaloids in honey from Echium plantagineum L. J. Agric. Food Chem. 1981, 29, 958–960. [Google Scholar] [CrossRef]
- Crout, D.H. Pyrrolizidine alkaloids. Biosynthesis of the angelate component of heliosupine. J. Chem. Soc. Perkin Trans. 1 1967, 13, 1233–1234. [Google Scholar] [CrossRef] [PubMed]
- Resch, J.F.; Rosberger, D.F.; Meinwald, J.; Appling, J.W. Biologically active pyrrolizidine alkaloids from the true forget-me-not, Myosotis scorpioides. J. Nat. Prod. 1982, 45, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Culvenor, C.C.J.; Edgar, J.A.; Frahn, J.L.; Smith, L.W.; Ulubelen, A.; Doganca, S. The structure of anadoline. Aust. J. Chem. 1975, 28, 173–178. [Google Scholar] [CrossRef]
- Ulubelen, A.; Doganca, S. Anadoline, a new senecio alkaloid from Symphytum orientale. Tetrahedron Lett. 1970, 11, 2583–2585. [Google Scholar] [CrossRef]
- Crowley, H.C.; Culvenor, C.C.J. Alkaloids of Cynoglossum latifolium R.Br. Latifoline and 7-Angelylretronecine. Aust. J. Chem. 1962, 15, 139–144. [Google Scholar] [CrossRef]
- Hagglund, K.M.; L’Empereur, K.M.; Roby, M.R.; Stermitz, F.R. Latifoline and Latifoline-N-Oxide from Hackelia floribunda, the Western False Forget-Me-Not. J. Nat. Prod. 1985, 48, 638–639. [Google Scholar] [CrossRef]
- Roitman, J.N.; Wong, R.Y. Revised Absolute Configurations of Latifolic Acid and the Pyrrolizidine Alkaloid Latifoline. Aust. J. Chem. 1988, 41, 1781–1787. [Google Scholar] [CrossRef]
- L’Empereur, K.M.; Li, Y.; Stermitz, F.R.; Crabtree, L. Pyrrolizidine Alkaloids from Hackelia californica and Gnophaela latipennis, an H. californica-Hosted Arctiid Moth. J. Nat. Prod. 1989, 52, 360–366. [Google Scholar] [CrossRef]
- Frolich, C.; Hartmann, T.; Ober, D. Tissue distribution and biosynthesis of 1,2-saturated pyrrolizidine alkaloids in Phalaenopsis hybrids (Orchidaceae). Phytochemistry 2006, 67, 1493–1502. [Google Scholar] [CrossRef]
- Luning, B.; Trankner, H.; Brandange, S. Studies on orchidaceae alkaloids V. A new alkaloid from Phalaenopsis amabilis Bl. Acta Chem. Scand. 1966, 20, 2011. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, X.L.; Wang, C.J.; Wang, Y.S.; Xiao, F.; Shan, L.H.; Guo, Z.Y.; Weng, J. Pyrrolizidine alkaloids from Liparis nervosa with inhibitory activities against LPS-induced NO production in RAW264.7 macrophages. Phytochemistry 2013, 93, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T.; Witte, L. Pyrrolizidine alkaloids: Chemical, biological and chemoecological aspects. In Alkaloids: Chemical and Biological Perspectives; Pergamon Press: Oxford, UK, 1995; Volume 9, pp. 155–233. [Google Scholar]
- Klásek, A.; Sedmera, P.; Boeva, A.; Šantavý, F. Pyrrolizidine alkaloids. XX. Nemorensine, an alkaloid from Senecio nemorensis L. Collect. Czech. Chem. Commun. 1973, 38, 2504–2512. [Google Scholar] [CrossRef]
- Langel, D.; Ober, D.; Pelser, P. The evolution of pyrrolizidine alkaloid biosynthesis and diversity in the Senecioneae. Phytochem. Rev. 2011, 10, 3–74. [Google Scholar] [CrossRef]
- Culvenor, C.C.J.; Smith, L.W.; Willing, R.I. Madurensine, a macrocyclic pyrrolizidine diester with the secondary ester attachment at C-6. J. Chem. Soc. D 1970, 65–66. [Google Scholar] [CrossRef]
- Neuner-Jehle, N.; Nesvadba, H.; Spiteller, G. Application of mass spectrometry to structure elucidation of alkaloids, 6th center: Pyrrolizidine alkaloids from laburnum. Chem. Mon. 1965, 96, 321–338. [Google Scholar] [CrossRef]
- Stelljes, M.E.; Kelley, R.B.; Molyneux, R.J.; Seiber, J.N. GC-MS Determination of Pyrrolizidine Alkaloids in Four Senecio Species. J. Nat. Prod. 1991, 54, 759–773. [Google Scholar] [CrossRef]
- El-Shazly, A.; Wink, M. Diversity of Pyrrolizidine Alkaloids in the Boraginaceae Structures, Distribution, and Biological Properties. Diversity 2014, 6, 188–282. [Google Scholar] [CrossRef] [Green Version]
- Mattocks, A.R.; Jukes, R. Improved field tests for toxic pyrrolizidine alkaloids. J. Nat. Prod. 1987, 50, 161–166. [Google Scholar] [CrossRef]
- Rozhon, W.; Kammermeier, L.; Schramm, S.; Towfique, N.; Adebimpe Adedeji, N.; Adesola Ajayi, S.; Poppenberger, B. Quantification of the Pyrrolizidine Alkaloid Jacobine in Crassocephalum crepidioides by Cation Exchange High-Performance Liquid Chromatography. Phytochem. Anal. 2017, 29, 48–58. [Google Scholar] [CrossRef]
- Joosten, L.; Cheng, D.; Mulder, P.P.; Vrieling, K.; van Veen, J.A.; Klinkhamer, P.G. The genotype dependent presence of pyrrolizidine alkaloids as tertiary amine in Jacobaea vulgaris. Phytochemistry 2011, 72, 214–222. [Google Scholar] [CrossRef]
- Ehmke, A.; von Borstel, K.; Hartmann, T. Alkaloid N-oxides as transport and vacuolar storage compounds of pyrrolizidine alkaloids in Senecio vulgaris L. Planta 1988, 176, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Joppe, H.; Schmaus, G. Thesinine-4′-O-beta-D-glucoside the first glycosylated plant pyrrolizidine alkaloid from Borago officinalis. Phytochemistry 2002, 60, 399–402. [Google Scholar] [CrossRef]
- Koulman, A.; Seeliger, C.; Edwards, P.J.; Fraser, K.; Simpson, W.; Johnson, L.; Cao, M.; Rasmussen, S.; Lane, G.A. E/Z-Thesinine-O-4′-alpha-rhamnoside, pyrrolizidine conjugates produced by grasses (Poaceae). Phytochemistry 2008, 69, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, E.; Byerrum, R.U. Biosynthesis of lupanine from lysine and other labeled compounds. Biochem. Biophys. Res. Commun. 1962, 7, 58–61. [Google Scholar] [CrossRef]
- Nowacki, E.; Byerrum, R.U. A study on the biosynthesis of the Crotalaria alkaloids. Life Sci. 1962, 1, 157–161. [Google Scholar] [CrossRef]
- Khan, H.A.; Robins, D.J. Pyrrolizidine alkaloid biosynthesis; incorporation of 13C-labelled putrescines into retronecine. J. Chem. Soc. Chem. Commun. 1981, 146–147. [Google Scholar] [CrossRef]
- Khan, H.A.; Robins, D.J. Pyrrolizidine alkaloids: Evidence for N-(4-aminobutyl)-1,4-diaminobutane (homospermidine) as an intermediate in retronecine biosynthesis. J. Chem. Soc. Chem. Commun. 1981, 554–556. [Google Scholar] [CrossRef]
- Rana, J.; Robins, D.J. Application of 2H n.m.r. Spectroscopy to study the incorporation of 2H-labelled putrescines into the pyrrolizidine alkaloid retrorsine. J. Chem. Soc. Perkin Trans. 1 1986, 983–988. [Google Scholar] [CrossRef]
- Hughes, C.A.; Letcher, R.; Warren, F.L. 956. The Senecio alkaloids. Part XVI. The biosynthesis of the “necine” bases from carbon-14 precursors. J. Chem. Soc. 1964, 4974–4978. [Google Scholar] [CrossRef]
- Bottomley, W.; Gheissman, T.A. Pyrrolizidine alkaloids. The biosynthesis of retronecine. Phytochemistry 1964, 3, 357–360. [Google Scholar] [CrossRef]
- Bale, N.M.; Crout, D.H.G. Determination of the relative rates of incorporation of arginine and ornithine into retronecine during pyrrolizidine alkaloid biosynthesis. Phytochemistry 1975, 14, 2617–2622. [Google Scholar] [CrossRef]
- Robins, D.J.; Sweeney, J.R. Pyrrolizidine alkaloid biosynthesis. Incorporation of 14C-labelled precursors into retronecine. J. Chem. Soc. Perkin Trans. 1 1981, 3083–3086. [Google Scholar] [CrossRef]
- Robins, D.J.; Sweeney, J.R. Pyrrolizidine alkaloid biosynthesis: Derivation of retronecine from L-arginine and L-ornithine. Phytochemistry 1983, 22, 457–459. [Google Scholar] [CrossRef]
- Khan, H.A.; Robins, D.J. Pyrrolizidine alkaloid biosynthesis. Synthesis of 13C-labelled putrescines and their incorporation into retronecine. J. Chem. Soc. Perkin Trans. 1 1985, 101–105. [Google Scholar] [CrossRef]
- Grue-Soerensen, G.; Spenser, I.D. Biosynthesis of retronecine. J. Am. Chem. Soc. 1981, 103, 3208–3210. [Google Scholar] [CrossRef]
- Grue-Sørensen, G.; Spenser, I.D. The biosynthesis of retronecine. Can. J. Chem. 1982, 60, 643–662. [Google Scholar] [CrossRef] [Green Version]
- Kelly, H.A.; Robins, D.J. Pyrrolizidine alkaloid biosynthesis. Incorporation of 13C-labelled precursors into rosmarinine. J. Chem. Soc. Perkin Trans. 1 1987, 177–180. [Google Scholar] [CrossRef]
- Khan, H.A.; Robins, D.J. Pyrrolizidine alkaloid biosynthesis. Synthesis of 14C-labelled homospermidines and their incorporation into retronecine. J. Chem. Soc. Perkin Trans. 1 1985, 819–824. [Google Scholar] [CrossRef]
- Robins, D.J. Chapter 1 Biosynthesis of Pyrrolizidine and Quinolizidine Alkaloids. In The Alkaloids: Chemistry and Pharmacology; Cordell, G.A., Ed.; Academic Press: Cambridge, MA, USA, 1995; Volume 46, pp. 1–61. [Google Scholar]
- Böttcher, F.; Adolph, R.D.; Hartmann, T. Homospermidine synthase, the first pathway-specific enzyme in pyrrolizidine alkaloid biosynthesis. Phytochemistry 1993, 32, 679–689. [Google Scholar] [CrossRef]
- Ober, D.; Hartmann, T. Phylogenetic origin of a secondary pathway: The case of pyrrolizidine alkaloids. Plant Mol. Biol. 2000, 44, 445–450. [Google Scholar] [CrossRef]
- Krishna, R.G.; Wold, F. Post-translational modification of proteins. Adv. Enzymol. Relat. Areas Mol. Biol. 1993, 67, 265–298. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Lee, Y.B.; Joe, Y.A. Hypusine is essential for eukaryotic cell proliferation. Neurosignals 1997, 6, 115–123. [Google Scholar] [CrossRef]
- Ober, D.; Kaltenegger, E. Pyrrolizidine alkaloid biosynthesis, evolution of a pathway in plant secondary metabolism. Phytochemistry 2009, 70, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Gill, G.P.; Bryant, C.J.; Fokin, M.; Huege, J.; Fraser, K.; Jones, C.; Cao, M.; Faville, M.J. Low pyrrolizidine alkaloid levels in perennial ryegrass is associated with the absence of a homospermidine synthase gene. BMC Plant Biol. 2018, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Livshultz, T.; Kaltenegger, E.; Straub, S.C.K.; Weitemier, K.; Hirsch, E.; Koval, K.; Mema, L.; Liston, A. Evolution of pyrrolizidine alkaloid biosynthesis in Apocynaceae: Revisiting the defence de-escalation hypothesis. New Phytol. 2018, 218, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Reimann, A.; Nurhayati, N.; Backenkohler, A.; Ober, D. Repeated evolution of the pyrrolizidine alkaloid-mediated defense system in separate angiosperm lineages. Plant Cell 2004, 16, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Ober, D.; Harms, R.; Witte, L.; Hartmann, T. Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties of deoxyhypusine synthase except binding the eIF5A precursor protein. J. Biol. Chem. 2003, 278, 12805–12812. [Google Scholar] [CrossRef] [PubMed]
- Ober, D.; Hartmann, T. Deoxyhypusine synthase from tobacco. cDNA isolation, characterization, and bacterial expression of an enzyme with extended substrate specificity. J. Biol. Chem. 1999, 274, 32040–32047. [Google Scholar] [CrossRef]
- Moll, S.; Anke, S.; Kahmann, U.; Hansch, R.; Hartmann, T.; Ober, D. Cell-specific expression of homospermidine synthase, the entry enzyme of the pyrrolizidine alkaloid pathway in Senecio vernalis, in comparison with its ancestor, deoxyhypusine synthase. Plant Physiol. 2002, 130, 47–57. [Google Scholar] [CrossRef]
- Anke, S.; Niemuller, D.; Moll, S.; Hansch, R.; Ober, D. Polyphyletic origin of pyrrolizidine alkaloids within the Asteraceae. Evidence from differential tissue expression of homospermidine synthase. Plant Physiol. 2004, 136, 4037–4047. [Google Scholar] [CrossRef]
- Anke, S.; Gonde, D.; Kaltenegger, E.; Hansch, R.; Theuring, C.; Ober, D. Pyrrolizidine alkaloid biosynthesis in Phalaenopsis orchids: Developmental expression of alkaloid-specific homospermidine synthase in root tips and young flower buds. Plant Physiol. 2008, 148, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Niemuller, D.; Reimann, A.; Ober, D. Distinct cell-specific expression of homospermidine synthase involved in pyrrolizidine alkaloid biosynthesis in three species of the boraginales. Plant Physiol. 2012, 159, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L. The evolution of functionally novel proteins after gene duplication. Proc. Biol. Sci. 1994, 256, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Evolution by gene duplication: An update. Trends Ecol. Evol. 2003, 18, 292–298. [Google Scholar] [CrossRef]
- Abdelhady, M.I.; Beuerle, T.; Ober, D. Homospermidine in transgenic tobacco results in considerably reduced spermidine levels but is not converted to pyrrolizidine alkaloid precursors. Plant Mol. Biol. 2009, 71, 145–155. [Google Scholar] [CrossRef]
- Robins, D.J. The pyrrolizidine alkaloids. Fortschr. Chem. Org. Naturst. 1982, 41, 115–203. [Google Scholar]
- Frolich, C.; Ober, D.; Hartmann, T. Tissue distribution, core biosynthesis and diversification of pyrrolizidine alkaloids of the lycopsamine type in three Boraginaceae species. Phytochemistry 2007, 68, 1026–1037. [Google Scholar] [CrossRef]
- Cheng, D.; Kirk, H.; Vrieling, K.; Mulder, P.P.; Klinkhamer, P.G. The relationship between structurally different pyrrolizidine alkaloids and western flower thrips resistance in F(2) hybrids of Jacobaea vulgaris and Jacobaea aquatica. J. Chem. Ecol. 2011, 37, 1071–1080. [Google Scholar] [CrossRef]
- Wesseling, A.M.; Demetrowitsch, T.J.; Schwarz, K.; Ober, D. Variability of Pyrrolizidine Alkaloid Occurrence in Species of the Grass Subfamily Pooideae (Poaceae). Front. Plant Sci. 2017, 8, 2046. [Google Scholar] [CrossRef]
- Dodson, C.D.; Stermitz, F.R. Pyrrolizidine Alkaloids from Borage (Borago officinalis) Seeds and Flowers. J. Nat. Prod. 1986, 49, 727–728. [Google Scholar] [CrossRef]
- O’Dowd, D.J.; Edgar, J.A. Seasonal dynamics in the pyrrolizidine alkaloids of Heliotropium europaeum. Aust. J. Ecol. 1989, 14, 95–105. [Google Scholar] [CrossRef]
- Birecka, H.; Catalfamo, J.L. Incorporation of assimilated carbon into aminoalcohols of Heliotropium spathulatum. Phytochemistry 1982, 21, 2645–2651. [Google Scholar] [CrossRef]
- Leete, E. Biosynthesis and metabolism of the tropane alkaloids. Planta Med. 1979, 36, 97–112. [Google Scholar] [CrossRef]
- Leete, E.; Murrill, J.B. Biosynthesis of the tiglic acid moiety of meteloidine in Datura meteloides. Tetrahedron Lett. 1967, 18, 1727–1730. [Google Scholar] [CrossRef]
- Leete, E. Biosynthetic conversion of α-methylbutyric acid to tiglic acid in Datura meteloides. Phytochemistry 1973, 12, 2203–2205. [Google Scholar] [CrossRef]
- Attygalle, A.B.; Wu, X.; Will, K.W. Biosynthesis of tiglic, ethacrylic, and 2-methylbutyric acids in a carabid beetle, Pterostichus (Hypherpes) californicus. J. Chem. Ecol. 2007, 33, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.G.; Bachhawat, B.K.; Coon, M.J. Tiglyl coenzyme A and alpha-methylacetoacetyl coenzyme A, intermediates in the enzymatic degradation of isoleucine. J. Biol. Chem. 1956, 218, 391–400. [Google Scholar]
- Mcgaw, B.A.; Woolley, J.G. The biosynthesis of angelic acid in Cynoglossum officinale. Phytochemistry 1979, 18, 1647–1649. [Google Scholar] [CrossRef]
- Hughes, C.; Warren, F.L. The Senecio alkaloids. Part XIV. The biological synthesis of the “necic” acids using carbon-14. J. Chem. Soc. 1962, 34–37. [Google Scholar] [CrossRef]
- Crout, D.H. Pyrrolizidine alkaloids. The biosynthesis of echimidinic acid. J. Chem. Soc. Perkin Trans. 1 1966, 21, 1968–1972. [Google Scholar] [CrossRef]
- Weber, S.; Eisenreich, W.; Bacher, A.; Hartmann, T. Pyrrolizidine alkaloids of the lycopsamine type: Biosynthesis of trachelanthic acid. Phytochemistry 1999, 50, 1005–1014. [Google Scholar] [CrossRef]
- Robins, D.J.; Bale, N.M.; Crout, D.H. Pyrrolizidine alkaloids. Biosynthesis of monocrotalic acid, the necic acid component of monocrotaline. J. Chem. Soc. Perkin Trans. 1 1974, 2082–2086. [Google Scholar] [CrossRef]
- Rao, P.G.; Zutshi, U.; Soni, A.; Atal, C.K. Studies on Incorporation of 14C–Labelled Precursors in Monocrotaline. Planta Med. 1979, 35, 279–282. [Google Scholar] [CrossRef]
- Devlin, J.A.; Robins, D.J. Pyrrolizidine alkaloids. Biosynthesis of trichodesmic acid. J. Chem. Soc. Perkin Trans. 1 1984, 1329–1332. [Google Scholar] [CrossRef]
- Crout, D.H.G.; Benn, M.H.; Imaseki, H.; Geissman, T.A. Pyrrolizidine alkaloids: The biosynthesis of seneciphyllic acid. Phytochemistry 1966, 5, 1–21. [Google Scholar] [CrossRef]
- Crout, D.H.; Smith, E.H.; Davies, N.M.; Whitehouse, D. Pyrrolizidine alkaloids. The biosynthesis of senecic acid. J. Chem. Soc. Perkin Trans. 1 1972, 5, 671–680. [Google Scholar] [CrossRef]
- Crout, D.H.G.; Davies, N.M.; Smith, E.H.; Whitehouse, D. Biosynthesis of the C10 necic acids of the pyrrolizidine alkaloids. J. Chem. Soc. D 1970, 635–636. [Google Scholar] [CrossRef]
- Davies, N.M.; Crout, D.H. Pyrrolizidine alkaloid biosynthesis. Relative rates of incorporation of the isomers of isoleucine into the necic acid component of senecionine. J. Chem. Soc. Perkin Trans. 1 1974, 2079–2082. [Google Scholar] [CrossRef]
- Bale, N.M.; Cahill, R.; Davies, N.M.; Mitchell, M.B.; Smith, E.H.; Crout, D.H.G. Biosynthesis of the necic acids of the pyrrolizidine alkaloids. Further investigations of the formation of senecic and isatinecic acids in Senecio species. J. Chem. Soc. Perkin Trans. 1 1978, 101–110. [Google Scholar] [CrossRef]
- Stirling, I.R.; Freer, I.K.A.; Robins, D.J. Pyrrolizidine Alkaloid Biosynthesis. Incorporation of 2-Aminobutanoic Acid Labeled with 13C or 2H into the Senecic Acid Portion of Rosmarinine and Senecionine. J. Chem. Soc. Perkin Trans. 1 1997, 1, 677–680. [Google Scholar] [CrossRef]
- Cahill, R.; Crout, D.H.G.; Gregorio, M.V.M.; Mitchell, M.B.; Muller, U.S. Pyrrolizidine alkaloid biosynthesis: Stereochemistry of the formation of isoleucine in Senecio species and of its conversion into necic acids. J. Chem. Soc. Perkin Trans. 1 1983, 173–180. [Google Scholar] [CrossRef]
- Binder, S. Branched-Chain Amino Acid Metabolism in Arabidopsis thaliana. Arabidopsis Book 2010, 8, e0137. [Google Scholar] [CrossRef]
- Sander, H.; Hartmann, T. Site of synthesis, metabolism and translocation of senecionine N-oxide in cultured roots of Senecio erucifolius. Plant Cell Tissue Organ Cult. 1989, 18, 19–31. [Google Scholar] [CrossRef]
- Hartmann, T.; Dierich, B. Chemical diversity and variation of pyrrolizidine alkaloids of the senecionine type: Biological need or coincidence? Planta 1998, 206, 443–451. [Google Scholar] [CrossRef]
- Kruse, L.H.; Stegemann, T.; Sievert, C.; Ober, D. Identification of a Second Site of Pyrrolizidine Alkaloid Biosynthesis in Comfrey to Boost Plant Defense in Floral Stage. Plant Physiol. 2017, 174, 47–55. [Google Scholar] [CrossRef]
- Hol, W.H. The effect of nutrients on pyrrolizidine alkaloids in Senecio plants and their interactions with herbivores and pathogens. Phytochem. Rev. 2011, 10, 119–126. [Google Scholar] [CrossRef]
- Kirk, H.; Vrieling, K.; Van Der Meijden, E.; Klinkhamer, P.G. Species by environment interactions affect pyrrolizidine alkaloid expression in Senecio jacobaea, Senecio aquaticus, and their hybrids. J. Chem. Ecol. 2010, 36, 378–387. [Google Scholar] [CrossRef]
- Kostenko, O.; Mulder, P.P.; Bezemer, T.M. Effects of root herbivory on pyrrolizidine alkaloid content and aboveground plant-herbivore-parasitoid interactions in Jacobaea vulgaris. J. Chem. Ecol. 2013, 39, 109–119. [Google Scholar] [CrossRef]
- Vrieling, K.; de Vos, H.; van Wijk, C.A. Genetic analysis of the concentrations of pyrrolizidine alkaloids in Senecio jacobaea. Phytochemistry 1993, 32, 1141–1144. [Google Scholar] [CrossRef]
- Van Dam, N.M.; Vrieling, K. Genetic variation in constitutive and inducible pyrrolizidine alkaloid levels in Cynoglossum officinale L. Oecologia 1994, 99, 374–378. [Google Scholar] [CrossRef]
- Macel, M.; Vrieling, K.; Klinkhamer, P.G. Variation in pyrrolizidine alkaloid patterns of Senecio jacobaea. Phytochemistry 2004, 65, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Pelser, P.B.; de Vos, H.; Theuring, C.; Beuerle, T.; Vrieling, K.; Hartmann, T. Frequent gain and loss of pyrrolizidine alkaloids in the evolution of Senecio section Jacobaea (Asteraceae). Phytochemistry 2005, 66, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Kirk, H.; Mulder, P.P.; Vrieling, K.; Klinkhamer, P.G. Pyrrolizidine alkaloid variation in shoots and roots of segregating hybrids between Jacobaea vulgaris and Jacobaea aquatica. New Phytol. 2011, 192, 1010–1023. [Google Scholar] [CrossRef]
- Wei, X.; Vrieling, K.; Mulder, P.P.J.; Klinkhamer, P.G.L. Methyl Jasmonate Changes the Composition and Distribution Rather than the Concentration of Defence Compounds: A Study on Pyrrolizidine Alkaloids. J. Chem. Ecol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sievert, C.; Beuerle, T.; Hollmann, J.; Ober, D. Single cell subtractive transcriptomics for identification of cell-specifically expressed candidate genes of pyrrolizidine alkaloid biosynthesis. Phytochemistry 2015, 117, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Mawla, A.M. Effect of certain elicitors on production of pyrrolizidine alkaloids in hairy root cultures of Echium rauwolfii. Pharmazie 2010, 65, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T.; Ehmke, A.; Eilert, U.; von Borstel, K.; Theuring, C. Sites of synthesis, translocation and accumulation of pyrrolizidine alkaloid N-oxides in Senecio vulgaris L. Planta 1989, 177, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T.; Toppel, G. Senecionine N-oxide, the primary product of pyrrolizidine alkaloid biosynthesis in root cultures of Senecio vulgaris. Phytochemistry 1987, 26, 1639–1643. [Google Scholar] [CrossRef]
- Van Dam, N.M.; Vuister, L.W.; Bergshoeff, C.; de Vos, H.; van Der Meijden, E. The “Raison D’etre” of pyrrolizidine alkaloids in Cynoglossum officinale: Deterrent effects against generalist herbivores. J. Chem. Ecol. 1995, 21, 507–523. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary Metabolites in Plant Defense-Mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Van der Meijden, E. Plant defence, an evolutionary dilemma: Contrasting effects of (specialist and generalist) herbivores and natural enemies. In Proceedings of the 9th International Symposium on Insect-Plant Relationships; Städler, E., Rowell-Rahier, M., Bauer, R., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1996; pp. 307–310. [Google Scholar]
- Hartmann, T. Chemical ecology of pyrrolizidine alkaloids. Planta 1999, 207, 483–495. [Google Scholar] [CrossRef]
- Boppré, M. Insects pharmacophagously utilizing defensive plant chemicals (Pyrrolizidine alkaloids). Naturwissenschaften 1986, 73, 17–26. [Google Scholar] [CrossRef]
- Cheng, D.; van der Meijden, E.; Mulder, P.P.; Vrieling, K.; Klinkhamer, P.G. Pyrrolizidine alkaloid composition influences cinnabar moth oviposition preferences in Jacobaea hybrids. J. Chem. Ecol. 2013, 39, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Vrieling, K.; Mulder, P.P.; Klinkhamer, P.G. Testing the generalist-specialist dilemma: The role of pyrrolizidine alkaloids in resistance to invertebrate herbivores in Jacobaea species. J. Chem. Ecol. 2015, 41, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Macel, M.; Bruinsma, M.; Dijkstra, S.M.; Ooijendijk, T.; Niemeyer, H.M.; Klinkhamer, P.G. Differences in effects of pyrrolizidine alkaloids on five generalist insect herbivore species. J. Chem. Ecol. 2005, 31, 1493–1508. [Google Scholar] [CrossRef]
- Leiss, K.A.; Choi, Y.H.; Abdel-Farid, I.B.; Verpoorte, R.; Klinkhamer, P.G. NMR metabolomics of thrips (Frankliniella occidentalis) resistance in Senecio hybrids. J. Chem. Ecol. 2009, 35, 219–229. [Google Scholar] [CrossRef]
- Dreyer, D.L.; Jones, K.C.; Molyneux, R.J. Feeding deterrency of some pyrrolizidine, indolizidine, and quinolizidine alkaloids towards pea aphid (Acyrthosiphon pisum) and evidence for phloem transport of indolizidine alkaloid swainsonine. J. Chem. Ecol. 1985, 11, 1045–1051. [Google Scholar] [CrossRef]
- Liu, X.; Klinkhamer, P.G.L.; Vrieling, K. The effect of structurally related metabolites on insect herbivores: A case study on pyrrolizidine alkaloids and western flower thrips. Phytochemistry 2017, 138, 93–103. [Google Scholar] [CrossRef]
- Nuringtyas, T.R.; Verpoorte, R.; Klinkhamer, P.G.L.; van Oers, M.M.; Leiss, K.A. Toxicity of Pyrrolizidine Alkaloids to Spodoptera exigua Using Insect Cell Lines and Injection Bioassays. J. Chem. Ecol. 2014, 40, 609–616. [Google Scholar] [CrossRef]
- Lindigkeit, R.; Biller, A.; Buch, M.; Schiebel, H.M.; Boppre, M.; Hartmann, T. The two facies of pyrrolizidine alkaloids: The role of the tertiary amine and its N-oxide in chemical defense of insects with acquired plant alkaloids. Eur. J. Biochem. 1997, 245, 626–636. [Google Scholar] [CrossRef]
- Von Borstel, K.; Hartmann, T. Selective uptake of pyrrolizidine N-oxides by cell suspension cultures from pyrrolizidine alkaloid producing plants. Plant Cell Rep. 1986, 5, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Vrieling, K.; Klinkhamer, P.G.L. Interactions between Plant Metabolites Affect Herbivores: A Study with Pyrrolizidine Alkaloids and Chlorogenic Acid. Front. Plant Sci. 2017, 8, 903. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Vrieling, K.; Klinkhamer, P.G.L. Phytochemical Background Mediates Effects of Pyrrolizidine Alkaloids on Western Flower Thrips. J. Chem. Ecol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Eisner, T.; Meinwald, J. The chemistry of sexual selection. Proc. Natl. Acad. Sci. USA 1995, 92, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J. Effects of pyrrolizidine alkaloids through different trophic levels. Phytochem. Rev. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- Martins, C.H.; Cunha, B.P.; Solferini, V.N.; Trigo, J.R. Feeding on Host Plants with Different Concentrations and Structures of Pyrrolizidine Alkaloids Impacts the Chemical-Defense Effectiveness of a Specialist Herbivore. PLoS ONE 2015, 10, e0141480. [Google Scholar] [CrossRef]
- Masters, A.R. Pyrrolizidine Alkaloids in Artificial Nectar Protect Adult Ithomiine Butterflies from a Spider Predator. Biotropica 1990, 22, 298–304. [Google Scholar] [CrossRef]
- Silva, K.L.; Trigo, J.R. Structure-activity relationships of pyrrolizidine alkaloids in insect chemical defense against the orb-weaving spider Nephila clavipes. J. Chem. Ecol. 2002, 28, 657–668. [Google Scholar] [CrossRef]
- Brown, K.S. Chemistry at the Solanaceae/Ithomiinae Interface. Ann. Mo. Bot. Garden 1987, 74, 359–397. [Google Scholar] [CrossRef]
- Boppré, M. Lepidoptera and pyrrolizidine alkaloids Exemplification of complexity in chemical ecology. J. Chem. Ecol. 1990, 16, 165–185. [Google Scholar] [CrossRef]
- Ehmke, A.; Witte, L.; Biller, A.; Hartmann, T. Sequestration, N-Oxidation and Transformation of Plant Pyrrolizidine Alkaloids by the Arctiid Moth Tyria jacobaeae L. Z. Naturforsch. C 1990, 45, 1185. [Google Scholar] [CrossRef]
- Hartmann, T.; Biller, A.; Witte, L.; Ernst, L.; Boppré, M. Transformation of plant pyrrolizidine alkaloids into novel insect alkaloids by Arctiid moths (Lepidoptera). Biochem. Syst. Ecol. 1990, 18, 549–554. [Google Scholar] [CrossRef]
- Kubitza, C.; Faust, A.; Gutt, M.; Gath, L.; Ober, D.; Scheidig, A.J. Crystal structure of pyrrolizidine alkaloid N-oxygenase from the grasshopper Zonocerus variegatus. Acta Crystallogr. D Struct. Biol. 2018, 74, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Macel, M.; Klinkhamer, P.G.L. Chemotype of Senecio jacobaea affects damage by pathogens and insect herbivores in the field. Evol. Ecol. 2010, 24, 237–250. [Google Scholar] [CrossRef]
- Joshi, J.; Vrieling, K. The Enemy Release and EICA hypothesis revisited: Incorporating the fundamental difference between specialist and generalist herbivores. Ecol. Lett. 2005, 8, 704–714. [Google Scholar] [CrossRef]
- Hol, W.H.; Van Veen, A. Pyrrolizidine alkaloids from Senecio jacobaea affect fungal growth. J. Chem. Ecol. 2002, 28, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.M.; Robinson, L.A.; Abdul-Sada, A.; Vanbergen, A.J.; Hodge, A.; Hartley, S.E. Arbuscular Mycorrhizal Fungi and Plant Chemical Defence: Effects of Colonisation on Aboveground and Belowground Metabolomes. J. Chem. Ecol. 2018, 44, 198–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reidinger, S.; Eschen, R.; Gange, A.C.; Finch, P.; Bezemer, T.M. Arbuscular mycorrhizal colonization, plant chemistry, and aboveground herbivory on Senecio jacobaea. Acta Oecol. 2012, 38, 8–16. [Google Scholar] [CrossRef]
- Irmer, S.; Podzun, N.; Langel, D.; Heidemann, F.; Kaltenegger, E.; Schemmerling, B.; Geilfus, C.M.; Zorb, C.; Ober, D. New aspect of plant-rhizobia interaction: Alkaloid biosynthesis in Crotalaria depends on nodulation. Proc. Natl. Acad. Sci. USA 2015, 112, 4164–4169. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Wittke, C.; Lederer, I.; Klier, B.; Kleinwachter, M.; Selmar, D. Interspecific transfer of pyrrolizidine alkaloids: An unconsidered source of contaminations of phytopharmaceuticals and plant derived commodities. Food Chem. 2016, 213, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Mathon, C.; Edder, P.; Bieri, S.; Christen, P. Survey of pyrrolizidine alkaloids in teas and herbal teas on the Swiss market using HPLC-MS/MS. Anal. Bioanal. Chem. 2014, 406, 7345–7354. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, M.; Prikerznik, M.; Kreft, S. FTIR spectroscopy as a tool to detect contamination of rocket (Eruca sativa and Diplotaxis tenuifolia) salad with common groundsel (Senecio vulgaris) leaves. J. Sci. Food Agric. 2017, 97, 2238–2244. [Google Scholar] [CrossRef] [PubMed]
- Dubecke, A.; Beckh, G.; Lullmann, C. Pyrrolizidine alkaloids in honey and bee pollen. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Beuerle, T.; Buhringer, M.; Denner, M.; Trost, D.; von der Ohe, K.; Bhavanam, V.B.; Schreier, P. Pyrrolizidine alkaloids in honey: Risk analysis by gas chromatography-mass spectrometry. Mol. Nutr. Food Res. 2008, 52, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Wittig, M.; Reinhard, A.; von der Ohe, K.; Blacquiere, T.; Raezke, K.P.; Michel, R.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in honey: Comparison of analytical methods. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Betz, J.M.; Eppley, R.M.; Taylor, W.C.; Andrzejewski, D. Determination of pyrrolizidine alkaloids in commercial comfrey products (Symphytum sp.). J. Pharm. Sci. 1994, 83, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.A.; Colegate, S.M.; Boppre, M.; Molyneux, R.J. Pyrrolizidine alkaloids in food: A spectrum of potential health consequences. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.S.; Pereira, T.N.; Reilly, P.E.; Seawright, A.A. Pyrrolizidine alkaloids in human diet. Mutat. Res. 1999, 443, 53–67. [Google Scholar] [CrossRef]
- Xia, Q.; Ma, L.; He, X.; Cai, L.; Fu, P.P. 7-Glutathione pyrrole adduct: A potential DNA reactive metabolite of pyrrolizidine alkaloids. Chem. Res. Toxicol. 2015, 28, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Xia, Q.; Lin, G.; Chou, M.W. Pyrrolizidine alkaloids—Genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab. Rev. 2004, 36, 1–55. [Google Scholar] [CrossRef]
- Kim, H.Y.; Stermitz, F.R.; Coulombe, R.A., Jr. Pyrrolizidine alkaloid-induced DNA-protein cross-links. Carcinogenesis 1995, 16, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhao, Y.; Von Tungeln, L.S.; Doerge, D.R.; Lin, G.; Cai, L.; Fu, P.P. Pyrrolizidine alkaloid-derived DNA adducts as a common biological biomarker of pyrrolizidine alkaloid-induced tumorigenicity. Chem. Res. Toxicol. 2013, 26, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Huxtable, R.J.; Yan, C.C.; Wild, S.; Maxwell, S.; Cooper, R. Physicochemical and metabolic basis for the differing neurotoxicity of the pyrrolizidine alkaloids, trichodesmine and monocrotaline. Neurochem. Res. 1996, 21, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Yang, Y.C.; Xia, Q.; Chou, M.W.; Cui, Y.Y.; Lin, G. Pyrrolizidine Alkaloids -Tumorigenic Components in Chinese Herbal Medicines and Dietary Supplements. J. Food Drug Anal. 2002, 10, 198–211. [Google Scholar]
- FAO/WHO. Discussion paper on pyrrolizidine alkaloids, Joint FAO/WHO food standards programme. In CODEX Committee on Contaminants in Foods, 5th ed.; FAO: The Hague, The Netherlands, 2011. [Google Scholar]
- Xia, Q.; Chou, M.W.; Kadlubar, F.F.; Chan, P.C.; Fu, P.P. Human liver microsomal metabolism and DNA adduct formation of the tumorigenic pyrrolizidine alkaloid, riddelliine. Chem. Res. Toxicol. 2003, 16, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Mattocks, A.R. Toxicity of pyrrolizidine alkaloids. Nature 1968, 217, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Mattocks, A.R. Hepatotoxic effects due to pyrrolizidine alkaloid N-oxides. Xenobiotica 1971, 1, 563–565. [Google Scholar] [CrossRef]
- White, I.N.; Mattocks, A.R. Some factors affecting the conversion of pyrrolizidine alkaloids to N-oxides and to pyrrolic derivatives in vitro. Xenobiotica 1971, 1, 503–505. [Google Scholar] [CrossRef]
- Lin, G.; Cui, Y.Y.; Hawes, E.M. Microsomal formation of a pyrrolic alcohol glutathione conjugate of clivorine. Firm evidence for the formation of a pyrrolic metabolite of an otonecine-type pyrrolizidine alkaloid. Drug Metab. Dispos. 1998, 26, 181–184. [Google Scholar]
- Mattocks, A.R.; White, I.N. The conversion of pyrrolizidine alkaloids to N-oxides and to dihydropyrrolizine derivatives by rat-liver microsomes in vitro. Chem. Biol. Interact. 1971, 3, 383–396. [Google Scholar] [CrossRef]
- Edgar, J.A.; Molyneux, R.J.; Colegate, S.M. Pyrrolizidine Alkaloids: Potential Role in the Etiology of Cancers, Pulmonary Hypertension, Congenital Anomalies, and Liver Disease. Chem. Res. Toxicol. 2015, 28, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P. Pyrrolizidine Alkaloids: Metabolic Activation Pathways Leading to Liver Tumor Initiation. Chem. Res. Toxicol. 2017, 30, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xia, Q.; Ruan, J.; Fu, P.P.; Lin, G. Hepatotoxicity and tumorigenicity induced by metabolic activation of pyrrolizidine alkaloids in herbs. Curr. Drug Metab. 2011, 12, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Nigra, L.; Huxtable, R.J. Hepatic glutathione concentrations and the release of pyrrolic metabolites of the pyrrolizidine alkaloid, monocrotaline, from the isolated perfused liver. Toxicon 1992, 30, 1195–1202. [Google Scholar] [CrossRef]
- Pereira, T.N.; Webb, R.I.; Reilly, P.E.; Seawright, A.A.; Prakash, A.S. Dehydromonocrotaline generates sequence-selective N-7 guanine alkylation and heat and alkali stable multiple fragment DNA crosslinks. Nucleic Acids Res. 1998, 26, 5441–5447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Li, L.; Zhong, D.; Shen, S.; Zheng, J.; Chen, X. 9-Glutathionyl-6,7-dihydro-1-hydroxymethyl-5H-pyrrolizine Is the Major Pyrrolic Glutathione Conjugate of Retronecine-Type Pyrrolizidine Alkaloids in Liver Microsomes and in Rats. Chem. Res. Toxicol. 2016, 29, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.A.; Seymour, J.L.; Hsia, M.T.; Allen, J.R. Covalent interaction of dehydroretronecine, a carcinogenic metabolite of the pyrrolizidine alkaloid monocrotaline, with cysteine and glutathione. Cancer Res. 1977, 37, 3141–3144. [Google Scholar] [PubMed]
- Dueker, S.R.; Lame, M.W.; Jones, A.D.; Morin, D.; Segall, H.J. Glutathione conjugation with the pyrrolizidine alkaloid, jacobine. Biochem. Biophys. Res. Commun. 1994, 198, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Liu, T.; Wang, Z. Pyrrolizidine alkaloid clivorine induced oxidative injury on primary cultured rat hepatocytes. Hum. Exp. Toxicol. 2010, 29, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Sheng, Y.; Jiang, P.; Ji, L.; Xia, Y.; Min, Y.; Wang, Z. The gender-dependent difference of liver GSH antioxidant system in mice and its influence on isoline-induced liver injury. Toxicology 2011, 280, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Chen, Y.; Wang, Z.Y.; Ji, L.L.; Wang, Z.T. Pyrrolizidine alkaloid isoline-induced oxidative injury in various mouse tissues. Exp. Toxicol. Pathol. 2010, 62, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Hsu, I.C.; Allen, J.R.; Chesney, C.F. Identification and toxicological effects of dehydroretronecine, a metabolite of monocrotaline. Proc. Soc. Exp. Biol. Med. 1973, 144, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Jago, M.V.; Edgar, J.A.; Smith, L.W.; Culvenor, C.C. Metabolic conversion of heliotridine-based pyrrolizidine alkaloids to dehydroheliotridine. Mol. Pharmacol. 1970, 6, 402–406. [Google Scholar] [PubMed]
- Fashe, M.M.; Juvonen, R.O.; Petsalo, A.; Rahnasto-Rilla, M.; Auriola, S.; Soininen, P.; Vepsalainen, J.; Pasanen, M. Identification of a new reactive metabolite of pyrrolizidine alkaloid retrorsine: (3H-pyrrolizin-7-yl)methanol. Chem. Res. Toxicol. 2014, 27, 1950–1957. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Mei, N.; Fu, P.P. Genotoxicity of pyrrolizidine alkaloids. J. Appl. Toxicol. 2010, 30, 183–196. [Google Scholar] [CrossRef]
- Yang, Y.C.; Yan, J.; Doerge, D.R.; Chan, P.C.; Fu, P.P.; Chou, M.W. Metabolic activation of the tumorigenic pyrrolizidine alkaloid, riddelliine, leading to DNA adduct formation in vivo. Chem. Res. Toxicol. 2001, 14, 101–109. [Google Scholar] [CrossRef]
- Fashe, M.M.; Juvonen, R.O.; Petsalo, A.; Rasanen, J.; Pasanen, M. Species-Specific Differences in the in Vitro Metabolism of Lasiocarpine. Chem. Res. Toxicol. 2015, 28, 2034–2044. [Google Scholar] [CrossRef]
- Lin, G.; Cui, Y.Y.; Liu, X.Q. Gender differences in microsomal metabolic activation of hepatotoxic clivorine in rat. Chem. Res. Toxicol. 2003, 16, 768–774. [Google Scholar] [CrossRef]
- Lin, G.; Tang, J.; Liu, X.Q.; Jiang, Y.; Zheng, J. Deacetylclivorine: A gender-selective metabolite of clivorine formed in female Sprague-Dawley rat liver microsomes. Drug Metab. Dispos. 2007, 35, 607–613. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Li, H.; Wang, X.; Chen, Y.; Guo, X.; Peng, Y.; Zheng, J. A Difference in Internal Exposure Makes Newly Weaned Mice More Susceptible to the Hepatotoxicity of Retrorsine Than Adult Mice. Chem. Res. Toxicol. 2018. [Google Scholar] [CrossRef]
- Williams, D.E.; Reed, R.L.; Kedzierski, B.; Dannan, G.A.; Guengerich, F.P.; Buhler, D.R. Bioactivation and detoxication of the pyrrolizidine alkaloid senecionine by cytochrome P-450 enzymes in rat liver. Drug Metab. Dispos. 1989, 17, 387–392. [Google Scholar] [PubMed]
- Williams, D.E.; Reed, R.L.; Kedzierski, B.; Ziegler, D.M.; Buhler, D.R. The role of flavin-containing monooxygenase in the N-oxidation of the pyrrolizidine alkaloid senecionine. Drug Metab. Dispos. 1989, 17, 380–386. [Google Scholar] [PubMed]
- Chou, M.W.; Wang, Y.P.; Yan, J.; Yang, Y.C.; Beger, R.D.; Williams, L.D.; Doerge, D.R.; Fu, P.P. Riddelliine N-oxide is a phytochemical and mammalian metabolite with genotoxic activity that is comparable to the parent pyrrolizidine alkaloid riddelliine. Toxicol. Lett. 2003, 145, 239–247. [Google Scholar] [CrossRef]
- Wang, Y.P.; Yan, J.; Fu, P.P.; Chou, M.W. Human liver microsomal reduction of pyrrolizidine alkaloid N-oxides to form the corresponding carcinogenic parent alkaloid. Toxicol. Lett. 2005, 155, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xia, Q.; Chou, M.W.; Fu, P.P. Metabolic activation of retronecine and retronecine N-oxide - formation of DHP-derived DNA adducts. Toxicol. Ind. Health 2008, 24, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Huan, J.Y.; Miranda, C.L.; Buhler, D.R.; Cheeke, P.R. Species differences in the hepatic microsomal enzyme metabolism of the pyrrolizidine alkaloids. Toxicol. Lett. 1998, 99, 127–137. [Google Scholar] [CrossRef]
- Chung, W.G.; Buhler, D.R. Major factors for the susceptibility of guinea pig to the pyrrolizidine alkaloid jacobine. Drug Metab. Dispos. 1995, 23, 1263–1267. [Google Scholar] [PubMed]
- Dueker, S.R.; Lame, M.W.; Morin, D.; Wilson, D.W.; Segall, H.J. Guinea pig and rat hepatic microsomal metabolism of monocrotaline. Drug Metab. Dispos. 1992, 20, 275–280. [Google Scholar]
- Dueker, S.R.; Lame, M.W.; Segall, H.J. Hydrolysis of pyrrolizidine alkaloids by guinea pig hepatic carboxylesterases. Toxicol. Appl. Pharmacol. 1992, 117, 116–121. [Google Scholar] [CrossRef]
- Tang, J.; Akao, T.; Nakamura, N.; Wang, Z.T.; Takagawa, K.; Sasahara, M.; Hattori, M. In vitro metabolism of isoline, a pyrrolizidine alkaloid from Ligularia duciformis, by rodent liver microsomal esterase and enhanced hepatotoxicity by esterase inhibitors. Drug Metab. Dispos. 2007, 35, 1832–1839. [Google Scholar] [CrossRef]
- He, Y.Q.; Yang, L.; Liu, H.X.; Zhang, J.W.; Liu, Y.; Fong, A.; Xiong, A.Z.; Lu, Y.L.; Yang, L.; Wang, C.H.; et al. Glucuronidation, a new metabolic pathway for pyrrolizidine alkaloids. Chem. Res. Toxicol. 2010, 23, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Liao, C.; Ye, Y.; Lin, G. Lack of metabolic activation and predominant formation of an excreted metabolite of nontoxic platynecine-type pyrrolizidine alkaloids. Chem. Res. Toxicol. 2014, 27, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, R.; Ansari, A.A.; Vankar, Y.D. Recent developments in design and synthesis of bicyclic azasugars, carbasugars and related molecules as glycosidase inhibitors. Chem. Soc. Rev. 2013, 42, 5102–5118. [Google Scholar] [CrossRef] [PubMed]
- Winchester, B.; Fleet, G.W.J. Amino-sugar glycosidase inhibitors: Versatile tools for glycobiologists. Glycobiology 1992, 2, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Nash, R.J.; Molyneux, R.J.; Fleet, G.W.J. Sugar-mimic glycosidase inhibitors: Natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron 2000, 11, 1645–1680. [Google Scholar] [CrossRef]
- Wong, C.-H.; Halcomb, R.L.; Ichikawa, Y.; Kajimoto, T. Enzymes in Organic Synthesis: Application to the Problems of Carbohydrate Recognition (Part 2). Angew. Chem. Int. Ed. 1995, 34, 521–546. [Google Scholar] [CrossRef]
- Davies, G.J.; Gloster, T.M.; Henrissat, B. Recent structural insights into the expanding world of carbohydrate-active enzymes. Curr. Opin. Struct. Biol. 2005, 15, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Compain, P.; Bodlenner, A. The multivalent effect in glycosidase inhibition: A new, rapidly emerging topic in glycoscience. ChemBioChem 2014, 15, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Asano, N. Sugar-mimicking glycosidase inhibitors: Bioactivity and application. Cell. Mol. Life Sci. 2009, 66, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Wrodnigg, T.M.; Steiner, A.J.; Ueberbacher, B.J. Natural and synthetic iminosugars as carbohydrate processing enzyme inhibitors for cancer therapy. Anticancer Agents Med. Chem. 2008, 8, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Rempel, B.P.; Withers, S.G. Covalent inhibitors of glycosidases and their applications in biochemistry and biology. Glycobiology 2008, 18, 570–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, A.A.; Fleet, G.W.; Asano, N.; Molyneux, R.J.; Nash, R.J. Polyhydroxylated alkaloids - natural occurrence and therapeutic applications. Phytochemistry 2001, 56, 265–295. [Google Scholar] [CrossRef]
- Vlietinck, A.J.; De Bruyne, T.; Apers, S.; Pieters, L.A. Plant-derived leading compounds for chemotherapy of human immunodeficiency virus (HIV) infection. Planta Med. 1998, 64, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Tropea, J.E.; Molyneux, R.J.; Kaushal, G.P.; Pan, Y.T.; Mitchell, M.; Elbein, A.D. Australine, a pyrrolizidine alkaloid that inhibits amyloglucosidase and glycoprotein processing. Biochemistry 1989, 28, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Kano, E.; Adachi, I.; Molyneux, R.J.; Watson, A.A.; Nash, R.J.; Fleet, G.W.J.; Wormald, M.R.; Kizu, H.; Ikeda, K.; et al. Australine and related alkaloids: Easy structural confirmation by 13C NMR spectral data and biological activities. Tetrahedron 2003, 14, 325–331. [Google Scholar] [CrossRef]
- Nash, R.J.; Fellows, L.E.; Dring, J.V.; Fleet, G.W.J.; Derome, A.E.; Hamor, T.A.; Scofield, A.M.; Watkin, D.J. Isolation from alexaleiopetala and X-ray crystal structure of alexine, (1r,2r,3r,7s,8s)-3-hydroxymethyl-1,2,7-trihydroxypyrrolizidine [(2r,3r,4r,5s,6s)-2-hydroxymethyl-1-azabicyclo 3.3.0 octan-3,4,6-triol], a unique pyrrolizidine alkaloid. Tetrahedron Lett. 1988, 29, 2487–2490. [Google Scholar] [CrossRef]
- Nash, R.J.; Fellows, L.E.; Dring, J.V.; Fleet, G.W.J.; Girdhar, A.; Ramsden, N.G.; Peach, J.M.; Hegarty, M.P.; Scofield, A.M. Two alexines 3-hydroxymethyl-1,2,7-trihydroxypyrrolizidines from Castanospermum australe. Phytochemistry 1990, 29, 111–114. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Kondo, S.; Ikeda, T.; Ikeda, D.; Miura, K.; Hamada, M.; Takeuchi, T.; Umezawa, H. New antibiotics clazamycins A and B. J. Antibiot. 1979, 32, 762–764. [Google Scholar] [CrossRef]
- Dolak, L.A.; DeBoer, C. Clazamycin B is antibiotic 354. J. Antibiot. 1980, 33, 83–84. [Google Scholar] [CrossRef]
- Sugie, Y.; Hirai, H.; Kachi-Tonai, H.; Kim, Y.J.; Kojima, Y.; Shiomi, Y.; Sugiura, A.; Sugiura, A.; Suzuki, Y.; Yoshikawa, N.; et al. New pyrrolizidinone antibiotics CJ-16,264 and CJ-16,367. J. Antibiot. 2001, 54, 917–925. [Google Scholar] [CrossRef]
- Nakai, R.; Ogawa, H.; Asai, A.; Ando, K.; Agatsuma, T.; Matsumiya, S.; Akinaga, S.; Yamashita, Y.; Mizukami, T. UCS1025A, a novel antibiotic produced by Acremonium sp. J. Antibiot. 2000, 53, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Roeder, E. Medicinal plants in China containing pyrrolizidine alkaloids. Pharmazie 2000, 55, 711–726. [Google Scholar] [PubMed]
- Roeder, E.; Wiedenfeld, H. Plants containing pyrrolizidine alkaloids used in the Traditional Indian medicine—Including Ayurveda. Pharmazie 2013, 68, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Roeder, E.; Wiedenfeld, H. Pyrrolizidine alkaloids in plants used in the traditional medicine of Madagascar and the Mascarene islands. Pharmazie 2011, 66, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Roeder, E.; Wiedenfeld, H.; Edgar, J.A. Pyrrolizidine alkaloids in medicinal plants from North America. Pharmazie 2015, 70, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.M.; Roby, M.R.; Stermitz, F.R. Unsaturated Pyrrolizidines from Borage (Borago officinalis), a Common Garden Herb. J. Nat. Prod. 1984, 47, 747–748. [Google Scholar] [CrossRef]
- Luthy, J.; Brauchli, J.; Zweifel, U.; Schmid, P.; Schlatter, C. Pyrrolizidine alkaloids in medicinal plants of Boraginaceal: Borago officinalis L. and Pulmonaria officinalis L. Pharm. Acta Helv. 1984, 59, 242–246. [Google Scholar] [PubMed]
- Wretensjö, I.; Karlberg, B. Pyrrolizidine alkaloid content in crude and processed borage oil from different processing stages. J. Am. Oil Chem. Soc. 2003, 80, 963–970. [Google Scholar] [CrossRef]
- Vacillotto, G.; Favretto, D.; Seraglia, R.; Pagiotti, R.; Traldi, P.; Mattoli, L. A rapid and highly specific method to evaluate the presence of pyrrolizidine alkaloids in Borago officinalis seed oil. J. Mass Spectrom. 2013, 48, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Pelser, P.B.; Nordenstam, B.; Kadereit, J.W.; Watson, L.E. An ITS Phylogeny of Tribe Senecioneae (Asteraceae) and a New Delimitation of Senecio L. Taxon 2007, 56, 1077–1104. [Google Scholar] [CrossRef]
- Denton, O.A. Crassocephalum crepidioides (Benth.) S. Moore. In Plant Resources of Tropical Africa 2. Vegetables; Grubben, G.J.H., Denton, O.A., Eds.; PROTA Foundation: Wageningen, The Netherlands, 2004; pp. 226–228. [Google Scholar]
- Joshi, R.K. Study on essential oil composition of the roots of Crassocephalum crepidioides (benth.) S. Moore. J. Chin. Chem. Soc. 2014, 59, 2363–2365. [Google Scholar] [CrossRef]
- Bosch, C.H. Crassocephalum rubens (Juss. ex Jacq.) S.Moore. In Plant Resources of Tropical Africa 2. Vegetables; Grubben, G.J.H., Denton, O.A., Eds.; PROTA Foundation: Wageningen, The Netherlands, 2004; pp. 228–229. [Google Scholar]
- Dairo, F.; Adanlawo, I. Nutritional Quality of Crassocephalum crepidioides and Senecio biafrae. Pak. J. Nutr. 2007, 6, 35–39. [Google Scholar] [CrossRef]
- Nakamura, I.; Hossain, M.A. Factors affecting seed gemination and seedling emergence of redflower ragleaf (Crassocephalum crepidioides). Weed Biol. Manag. 2009, 9, 315–322. [Google Scholar] [CrossRef]
- Adedayo, B.C.; Oboh, G.; Oyeleye, S.I.; Ejakpovi, I.I.; Boligon, A.A.; Athayde, M.L. Blanching alters the phenolic constituents and in vitro antioxidant and anticholinesterases properties of fireweed (Crassocephalum crepidioides). J. Taibah Univ. Med. Sci. 2015, 10, 419–426. [Google Scholar] [CrossRef]
- Adjatin, A.; Dansi, A.; Badoussi, M.E.; Sanoussi, F.; Dansi, M.; Azokpota, P.; Ahissou, H.; Akouegninou, A.; Koffi, A.; Sanni, A. Proximate, mineral and vitamin C composition of vegetable Gbolo [Crassocephalum rubens (Juss. ex Jacq.) S. Moore and C. crepidioides (Benth.) S. Moore] in Benin. Int. J. Biol. Chem. Sci. 2013, 7, 319–331. [Google Scholar] [CrossRef]
- Adjatin, A.; Dansi, A.; Eze, S.; Assogba, P.; Dossou-Aminon, I.; Koffi, A.; Akoègninou, A.; Sanni, A. Ethnobotanical investigation and diversity of Gbolo (Crassocephalum rubens (Juss. ex Jacq.) S. Moore and Crassocephalum crepidioides (Benth.) S. Moore), a traditional leafy vegetable under domestication in Benin. Genet. Resour. Crop. Evol. 2012, 59, 1867–1881. [Google Scholar] [CrossRef]
- Dansi, A.; Adjatin, A.; Adoukonou-Sagbadja, H.; Faladé, V.; Yedomonhan, H.; Odou, D.; Dossou, B. Traditional leafy vegetables and their use in the Benin Republic. Genet. Resour. Crop. Evol. 2008, 55, 1239–1256. [Google Scholar] [CrossRef]
- Adjatin, A.; Dansi, A.; Badoussi, M.E.; Loko, L.; Dansi, M.; Azokpota, P.; Gbaguidi, F.; Ahissou, H.; Akoègninou, A.; Koffi, A.; et al. Phytochemical screening and toxicity studies of Crassocephalum rubens (Juss. ex Jacq.) S. Moore and Crassocephalum crepidioides (Benth.) S. Moore consumed as vegetable in Benin. J. Chem. Pharm. Res. 2013, 2, 1–13. [Google Scholar]
- Asada, Y.; Shiraishi, M.; Takeuchi, T.; Osawa, Y.; Furuya, T. Pyrrolizidine Alkaloids from Crassocephalum crepidioides. Planta Med. 1985, 51, 539–540. [Google Scholar] [CrossRef]
- Adegoke, E.A.; Akinsaya, A.; Naqvi, H.Z. Studies of Nigerian medicinal plants: A preliminary survey of plant alkaloid. J. West Afr. Sci. Assoc. 1968, 13, 13–33. [Google Scholar]
- Chao, C.Y.; Liu, W.H.; Wu, J.J.; Yin, M.C. Phytochemical profile, antioxidative and anti-inflammatory potentials of Gynura bicolor DC. J. Sci. Food Agric. 2015, 95, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Ren, B.R.; Min, Z.; Hu, Y.; Lu, C.G.; Wu, J.L.; Chen, J.; Sun, S. The anti-hyperglycemic effect of plants in genus Gynura Cass. Am. J. Chin. Med. 2009, 37, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Teoh, W.Y.; Sim, K.S.; Moses Richardson, J.S.; Abdul Wahab, N.; Hoe, S.Z. Antioxidant Capacity, Cytotoxicity, and Acute Oral Toxicity of Gynura bicolor. Evid. Based Complement. Altern. Med. 2013, 2013, 958407. [Google Scholar] [CrossRef] [PubMed]
- Teoh, W.Y.; Tan, H.P.; Ling, S.K.; Abdul Wahab, N.; Sim, K.S. Phytochemical investigation of Gynura bicolor leaves and cytotoxicity evaluation of the chemical constituents against HCT 116 cells. Nat. Prod. Res. 2016, 30, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Roeder, E.; Eckert, A.; Wiedenfeld, H. Pyrrolizidine alkaloids from Gynura divaricata. Planta Med. 1996, 62, 386. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, H.; Fang, L.X.; Li, W.L.; Verschaeve, L.; Wang, Z.T.; De Kimpe, N.; Mangelinckx, S. Detection and Toxicity Evaluation of Pyrrolizidine Alkaloids in Medicinal Plants Gynura bicolor and Gynura divaricata Collected from Different Chinese Locations. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Seow, L.J.; Beh, H.K.; Majid, A.M.; Murugaiyah, V.; Ismail, N.; Asmawi, M.Z. Anti-angiogenic activity of Gynura segetum leaf extracts and its fractions. J. Ethnopharmacol. 2011, 134, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Seow, L.J.; Beh, H.K.; Umar, M.I.; Sadikun, A.; Asmawi, M.Z. Anti-inflammatory and antioxidant activities of the methanol extract of Gynura segetum leaf. Int. Immunopharmacol. 2014, 23, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Yu, Y.C.; Ren, T.H.; Wu, J.G.; Jiang, Y.; Shen, L.G.; Zhang, J. Gynura root induces hepatic veno-occlusive disease: A case report and review of the literature. World J. Gastroenterol. 2007, 13, 1628–1631. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, G.; Teng, X.; Zhang, Z.; Pan, J.; Shou, Q.; Chen, M. Hematologic toxicity of Gynura segetum and effects on vascular endothelium in a rat model of hepatic veno-occlusive disease. Zhonghua Gan Zang Bing Za Zhi 2015, 23, 59–63. [Google Scholar] [CrossRef]
- Lin, G.; Wang, J.Y.; Li, N.; Li, M.; Gao, H.; Ji, Y.; Zhang, F.; Wang, H.; Zhou, Y.; Ye, Y.; et al. Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. J. Hepatol. 2011, 54, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Z.; Ji, T.; Bai, X.L.; Liang, L.; Wang, L.Y.; Chen, W.; Liang, T.B. Expression of MMP-9 in hepatic sinusoidal obstruction syndrome induced by Gynura segetum. J. Zhejiang Univ. Sci. B 2013, 14, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.T.; Roeder, E. Senecionine from Gynura segetum. Planta Med. 1984, 50, 362. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.Q.; Gu, G.M.; Wei, T.T. Studies on the alkaloids of Gynura segetum (Lour.) Merr. Yao Xue Xue Bao 1990, 25, 191–197. [Google Scholar] [PubMed]
- Qi, X.; Wu, B.; Cheng, Y.; Qu, H. Simultaneous characterization of pyrrolizidine alkaloids and N-oxides in Gynura segetum by liquid chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, R.T.; White, E.P.; Mortimer, P.H. Ryegrass staggers: Isolation of potent neurotoxins lolitrem A and lolitrem B from staggers-producing pastures. N. Z. Vet. J. 1981, 29, 189–190. [Google Scholar] [CrossRef]
- Lyons, P.C.; Plattner, R.D.; Bacon, C.W. Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science 1986, 232, 487–489. [Google Scholar] [CrossRef]
- Luo, H.; Xie, L.; Zeng, J.; Xie, J. Biosynthesis and Regulation of Bioprotective Alkaloids in the Gramineae Endophytic Fungi with Implications for Herbivores Deterrents. Curr. Microbiol. 2015, 71, 719–724. [Google Scholar] [CrossRef]
- Guerre, P. Ergot alkaloids produced by endophytic fungi of the genus Epichloe. Toxins 2015, 7, 773–790. [Google Scholar] [CrossRef]
- Wachenheim, D.E.; Blythe, L.L.; Craig, A.M. Characterization of rumen bacterial pyrrolizidine alkaloid biotransformation in ruminants of various species. Vet. Hum. Toxicol. 1992, 34, 513–517. [Google Scholar]




















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants. Molecules 2019, 24, 498. https://doi.org/10.3390/molecules24030498
Schramm S, Köhler N, Rozhon W. Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants. Molecules. 2019; 24(3):498. https://doi.org/10.3390/molecules24030498
Chicago/Turabian StyleSchramm, Sebastian, Nikolai Köhler, and Wilfried Rozhon. 2019. "Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants" Molecules 24, no. 3: 498. https://doi.org/10.3390/molecules24030498