Synthesis and Density Functional Theory Studies of Azirinyl and Oxiranyl Functionalized Isoindigo and (3Z,3’Z)-3,3’-(ethane-1,2-diylidene)bis(indolin-2-one) Derivatives
Abstract
:1. Introduction
2. Results
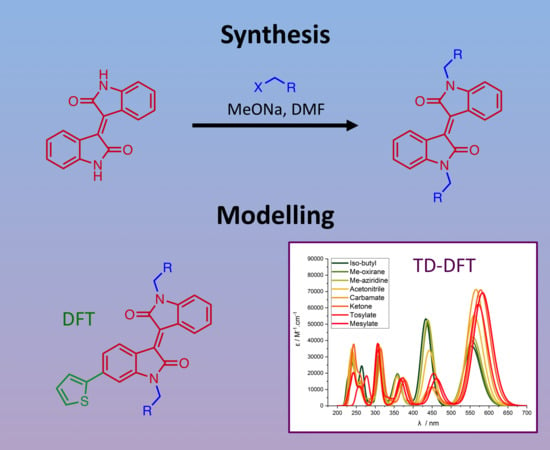
2.1. Chemical Synthesis and Characterisation
2.2. Computational Studies
3. Conclusions
4. Experimental Section
4.1. General Methods
4.2. Synthesis and Characterisation
4.2.1. General procedure for the synthesis of 7
4.2.2. General procedure for the synthesis of 6
4.3. Computational Method
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roncali, J. Molecular Bulk Heterojunctions: An Emerging Approach to Organic Solar Cells. Acc. Chem. Res. 2009, 42, 1719–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, B.; Kim, C.; Nguyen, T. Small Molecule Solution-Processed Bulk Heterojunction Solar Cells. Chem. Mater. 2011, 23, 470–482. [Google Scholar] [CrossRef]
- Hains, A.; Liang, Z.; Woodhouse, M.; Gregg, B. Molecular Semiconductors in Organic Photovoltaic Cells. Chem. Rev. 2010, 110, 6689–6735. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Hiszpanski, A.M.; Whittaker-Brooks, L.; Loo, Y.-L. Structure–Property Relationship Study of Substitution Effects on Isoindigo-Based Model Compounds as Electron Donors in Organic Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 14533–14542. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.A.; Stalder, R.; Abboud, K.A.; Risko, C.; Bredas, J.-L.; Reynolds, J.R. Understanding the Electronic Structure of Isoindigo in Conjugated Systems: A Combined Theoretical and Experimental Approach. Macromolecules 2013, 46, 8832–8844. [Google Scholar] [CrossRef]
- Stalder, R.; Mei, J.; Graham, K.R.; Estrada, L.A.; Reynolds, J.R. Isoindigo, a Versatile Electron-Deficient Unit For High-Performance Organic Electronics. Chem. Mater. 2014, 26, 664–678. [Google Scholar] [CrossRef]
- Mei, J.; Graham, K.R.; Stalder, R.; Reynolds, J.R. Synthesis of Isoindigo-Based Oligothiophenes for Molecular Bulk Heterojunction Solar Cells. Org. Lett. 2010, 12, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.A.; Liu, D.Y.; Salazar, D.H.; Dyer, A.L.; Reynolds, J.R. Poly[Bis-EDOTIsoindigo]: An Electroactive Polymer Applied to Electrochemical Supercapacitors. Macromolecules 2012, 45, 8211–8220. [Google Scholar] [CrossRef]
- Wang, E.; Mammo, W.; Andersson, M.R. 25th Anniversary Article: Isoindigo-Based Polymers and Small Molecules for Bulk Heterojunction Solar Cells and Field Effect Transistors. Adv. Mater. 2014, 26, 1801–1826. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, J.; Wang, J.; Liu, L.; Li, W.; Tian, H.; Zhang, X.; Xie, Z.; Geng, Y.; Wang, F. Dithienocarbazole and Isoindigo based Amorphous Low Bandgap Conjugated Polymers for Efficient Polymer Solar Cells. Adv. Mater. 2014, 26, 471–476. [Google Scholar] [CrossRef]
- Nishinaga, S.; Mori, H.; Nishihara, Y. Impact of Alkyl Side Chains on Thin-film Transistor Performances in Phenanthrodithiophene - Isoindigo Copolymers. Chem. Lett. 2015, 44, 998–1000. [Google Scholar] [CrossRef]
- Wang, E.; Ma, Z.; Zhang, Z.; Henriksson, P.; Inganas, O.; Zhang, F.; Andersson, M.R. An isoindigo-based low band gap polymer for efficient polymer solar cells with high photovoltage. Chem. Commun. 2011, 47, 4908–4910. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Khalili, G.; Willis, A.; Keller, P. Design and synthesis of new functionalized isoindigo and (3Z,3Z)-3,3-(ethane-1,2-diylidene)bis(indolin-2-one) derivatives. Mon. Fur Chem.-Chem. 2018, 149, 2103–2111. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Z.; Hou, R.; Huang, M.; Zhao, B.; Tan, S. The effect of the length of alkyl side-chains on the molecular aggregation and photovoltaic performance of the isoindigo based polymers. Dye. Pigment. 2017, 139, 403–411. [Google Scholar] [CrossRef]
- Grand, C.; Baek, S.; Lai, T.-H.; Deb, N.; Zajaczkowski, W.; Stalder, R.; Mullen, K.; Pisula, W.; Bucknall, D.G.; So, F.; et al. Structure–Property Relationships Directing Transport and Charge Separation in Isoindigo Polymers. Macromolecules 2016, 49, 4008–4022. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, H.; You, W. Quantitatively Analyzing the Influence of Side Chains on Photovoltaic Properties of PolymerFullerene Solar Cells. J. Phys. Chem. C 2010, 114, 16793–16800. [Google Scholar] [CrossRef]
- Mei, J.; Bao, Z. Side Chain Engineering in Solution-Processable Conjugated Polymers. Chem. Mater. 2014, 26, 604–615. [Google Scholar] [CrossRef]
- Ma, Z.; Geng, H.; Wang, D.; Shuai, Z. Influence of alkyl side-chain length on the carrier mobility in organic semiconductors: Herringbone vs. pi–pi stacking. J. Mater. Chem. C 2016, 4, 4546–4555. [Google Scholar] [CrossRef]
- Dang, D.; Chen, W.; Himmelberger, S.; Tao, Q.; Lundin, A.; Yang, R.; Zhu, W.; Salleo, A.; Muller, C.; Wang, E. Enhanced Photovoltaic Performance of Indacenodithiophene-Quinoxaline Copolymers by Side-Chain Modulation. Adv. Energy Mater. 2014, 4, 1400680. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 2014, 5, 5293. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, I.; Lai, T.-H.; Klump, E.D.; Goswami, S.; Schanze, K.S.; So, F. Effect of Polymer Side Chains on Charge Generation and Disorder in PBDTTPD Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 26999–27005. [Google Scholar] [CrossRef] [PubMed]
- Han, A.-R.; Lee, J.; Lee, H.R.; Lee, J.; Kang, S.-H.; Ahn, H.; Shin, T.J.; Oh, J.H.; Yang, C. Siloxane Side Chains: A Universal Tool for Practical Applications of Organic Field Effect Transistors. Macromolecules 2016, 49, 3739–3748. [Google Scholar] [CrossRef]
- Wang, P.-I.; Pisula, W.; Mullen, K.; Liaw, D.-J. Structurally defined nanographene-containing conjugated polymers for high quality dispersions and optoelectronic applications. Polym. Chem. 2016, 7, 6211–6219. [Google Scholar] [CrossRef] [Green Version]
- Salvatori, P.; Mosconi, E.; Wang, E.; Andersson, M.; Muccini, M.; De Angelis, F. Computational Modeling of Isoindigo-Based Polymers Used in Organic Solar Cells. J. Phys. Chem. C 2013, 117, 17940–17954. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.; Mosconi, E.; De Angelis, F.; Gratzel, M. A Computational Investigation of¨ Organic Dyes for Dye-Sensitized Solar Cells: Benchmark, Strategies, and Open Issues. J. Phys. Chem. C 2010, 114, 7205–7212. [Google Scholar] [CrossRef]
- Perpète, E.A.; Preat, J.; André, J.-M.; Jacquemin, D. An Ab Initio Study of the Absorption Spectra of Indirubin, Isoindigo, and Related Derivatives. J. Phys. Chem. A 2006, 110, 5629–5635. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Robinson, D. Comparison of the Transition Dipole Moments Calculated by TDDFT with High Level Wave Function Theory. J. Chem. Theory Comput. 2018, 14, 5303–5309. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.L. Natural transition orbitals. J. Chem. Phys. 2003, 118, 4775–4777. [Google Scholar] [CrossRef]
- Lowden, P. Aziridines and Epoxides in Organic Synthesis; Yudin, A., Ed.; Wiley-VCH: Weinheim, Germany, 2006; p. 399. [Google Scholar]
- Ismail, F.; Levitsky, D.; Dembitsky, V. Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem. 2009, 44, 3373–3387. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Wales, S.M.; Willis, A.C.; Keller, P.A. Ring-Opening and -Expansion of 2,2’-Biaziridine: Access to Diverse Enantiopure Linear and Bicyclic Vicinal Diamines. Org. Lett. 2014, 16, 4344–4347. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, B.; Guo, C.; Hong, W.; Meng, Y.; Li, Y. 3,3-(Ethane-1,2-diylidene)bis(indolin2-one) based conjugated polymers for organic thin film transistors. Chem. Commun. 2014, 50, 6509–6512. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Sudalai, A. N-Bromoamides as versatile catalysts for aziridination of olefins using chloramine-T. Tetrahedron Lett. 2003, 44, 989–992. [Google Scholar] [CrossRef]
- Ali, S.; Nikalje, M.; Sudalai, A. Pyridinium Hydrobromide Perbromide: A Versatile Catalyst for Aziridination of Olefins Using Chloramine-T. Org. Lett. 1999, 1, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.M.; Hendra, R.; Bremner, J.B.; Willis, A.C.; Lucantoni, L.; Avery, V.M.; Keller, P.A. Cascade Reactions of Indigo with Oxiranes and Aziridines: Efficient Access to Dihydropyrazinodiindoles and Sprio-oxazocinodiindoles. Org. Biomol. Chem. 2018, 16, 6006–6016. [Google Scholar] [CrossRef] [PubMed]
- Tanner, D. Chiral Aziridines—Their Synthesis and Use in Stereoselective Transformations. Angew. Chem. Int. Ed. 1994, 33, 599–619. [Google Scholar] [CrossRef]
- Panda, A.N.; Plasser, F.; Aquino, A.J.A.; Burghardt, I.; Lischka, H. Electronically Excited States in Poly(p-phenylenevinylene): Vertical Excitations and Torsional Potentials from High Level Ab Initio Calculations. J. Phys. Chem. A 2013, 117, 2181–2189. [Google Scholar] [CrossRef]
- Tehrani, K.A.; Van, T.N.; Karikomi, M.; Rottiers, M.; De Kimpe, N. Electron Transfer Induced Ring Opening of 2-(bromomethyl)aziridines by Magnesium in Methanol. Tetrahedron 2002, 58, 7145–7152. [Google Scholar] [CrossRef]
- D’hooghe, M.; Kerkaert, I.; Rottiers, M.; Norbert De Kimpe, N. Ring Opening Reactions of 1-arenesulfonyl-2-(bromomethyl)aziridines. Tetrahedron 2004, 60, 3637–3641. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3, 5, 7a–d, and 7f–i are available from the authors. |









| Entry | Compound | R | Yield % |
|---|---|---|---|
| 1 | 7a | propyl | 58 |
| 2 | 7b | isopropyl | 57 |
| 3 | 7c | ethyl | 59 |
| 4 | 7d | methyl | 60 |
| 5 | 7e |  | 57 |
| 6 | 7f |  | 53 |
| 7 | 7g |  | 54 |
| 8 | 7h |  | 56 |
| 9 | 7i |  | 52 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalili, G.; McCosker, P.M.; Clark, T.; Keller, P.A. Synthesis and Density Functional Theory Studies of Azirinyl and Oxiranyl Functionalized Isoindigo and (3Z,3’Z)-3,3’-(ethane-1,2-diylidene)bis(indolin-2-one) Derivatives. Molecules 2019, 24, 3649. https://doi.org/10.3390/molecules24203649
Khalili G, McCosker PM, Clark T, Keller PA. Synthesis and Density Functional Theory Studies of Azirinyl and Oxiranyl Functionalized Isoindigo and (3Z,3’Z)-3,3’-(ethane-1,2-diylidene)bis(indolin-2-one) Derivatives. Molecules. 2019; 24(20):3649. https://doi.org/10.3390/molecules24203649
Chicago/Turabian StyleKhalili, Gholamhossein, Patrick M. McCosker, Timothy Clark, and Paul A. Keller. 2019. "Synthesis and Density Functional Theory Studies of Azirinyl and Oxiranyl Functionalized Isoindigo and (3Z,3’Z)-3,3’-(ethane-1,2-diylidene)bis(indolin-2-one) Derivatives" Molecules 24, no. 20: 3649. https://doi.org/10.3390/molecules24203649