Ionothermal Synthesis of Cadmium Coordination Polymers: Ionic Liquid Effects on the Synthesis, Structural, and Thermal Characterization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ionothermal Synthesis
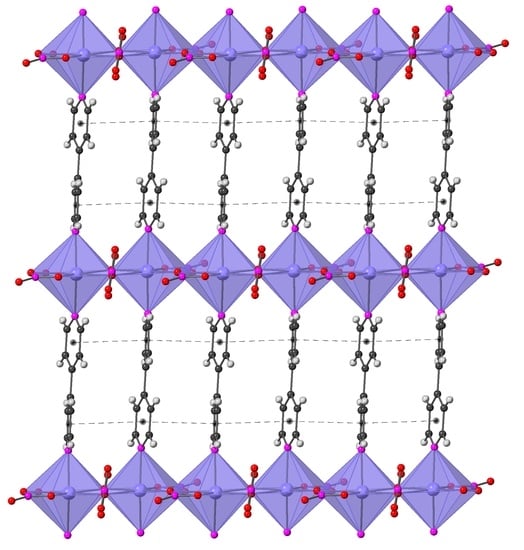
2.2. Crystal Structure
2.3. Thermal Characterization
3. Materials and Methods
3.1. Synthesis of Cd3(Ox)F2(Ina)2 (1)
3.2. Synthesis of Cd(4,4′-Bpy)(NO3)2 (2)
3.3. Single-crystal X-ray Diffraction Characterization
3.4. Physico-Chemical Characterization Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Cota, I.; Fernandez Martinez, F. Recent advances in the synthesis and applications of metal organic frameworks doped with ionic liquids for CO2 adsorption. Coord. Chem. Rev. 2017, 351, 189–204. [Google Scholar] [CrossRef]
- Lanchas, M.; Arcediano, S.; Beobide, G.; Castillo, O.; Luque, A.; Pérez-Yáñez, S. Towards multicomponent MOFs via solvent-free synthesis under conventional oven and microwave assisted heating. Inorg. Chem. Front. 2015, 2, 425–433. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.-L.; Pu, W.; Liu, P.; Liu, S.-X.; Li, Y.; Liu, X.-L.; Lu, Z.-X.; Zheng, L.-Y.; Cao, Q.-E. A hydrogel directly assembled from a copper metal–organic polyhedron for antimicrobial application. Chem. Commun. 2019, 55, 2206–2209. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Cheng, F.-F.; Xiong, W.-W.; Zhang, Q. New synthetic strategies to prepare metal—organic frameworks. Inorg. Chem. Front. 2018, 5, 2693–2708. [Google Scholar] [CrossRef]
- Liu, L.; Wei, H.; Zhang, L.; Li, J.; Dong, J. Ionothermal synthesis of the Metal-Organic Framework compound Cu3(BTC)2. In Studies in Surface Science and Catalysis; Gédéon, A., Massiani, P., Babonneau, F., Eds.; Elsevier Science: Paris, France, 2008; Volume 174, pp. 459–462. [Google Scholar]
- Martins, G.A.V.; Byrne, P.J.; Allan, P.; Teat, S.J.; Slawin, A.M.Z.; Li, Y.; Morris, R.E. The use of ionic liquids in the synthesis of zinc imidazolate frameworks. Dalton Trans. 2010, 39, 1758–1762. [Google Scholar] [CrossRef]
- Fischer, M.; Schwegler, J.; Paula, C.; Schulz, P.S.; Hartmann, M. Direct synthesis of non-breathing MIL-53(Al)(ht) from a terephthalate-based ionic liquid as linker precursor. Dalton Trans. 2016, 45, 18443–18446. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Xie, Z.-L.; Huang, X.-Y.; Xiao, X.-R. Ionothermal synthesis, crystal structure, and properties of an anionic two-dimensional cadmium metal organic framework based on paddle wheel-like cluster. Inorg. Chem. Commun. 2011, 14, 1001–1003. [Google Scholar] [CrossRef]
- Xie, Z.-L.; Feng, M.-L.; Tan, B.; Huang, X.-Y. The multifunctional roles of the ionic liquid [Bmim] [BF4] in the creation of cadmium metal–organic frameworks. CrystEngComm 2012, 14, 4894–4901. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Xu, L.; Jiao, H. Ionothermal synthesis, structures, properties of cobalt-1, 4-benzenedicarboxylate metal–organic frameworks. J. Solid State Chem. 2016, 238, 217–222. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Z.-H.; Wang, M.-M.; Xu, L.; Liu, B.; Jiao, H. Combination effect of ligands and ionic liquid components on the structure and properties of manganese metal–organic frameworks. CrystEngComm 2017, 19, 5402–5411. [Google Scholar] [CrossRef]
- Vaid, T.P.; Kelley, S.P.; Rogers, R.D. Structure-directing effects of ionic liquids in the ionothermal synthesis of metal–organic frameworks. IUCrJ 2017, 4, 380–392. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Wasserscheid, P.; Welton, T. Introduction. In Ionic Liquids in Synthesis; Wasserscheid, P., Welton, T., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 1–6. ISBN 978-3-527-62119-4. [Google Scholar]
- Holbrey, J.D.; Rogers, R.D.; Mantz, R.A.; Trulove, P.C.; Cocalia, V.A.; Visser, A.E.; Anderson, J.L.; Anthony, J.L.; Brennecke, J.F.; Maginn, E.J.; et al. Physicochemical Properties. In Ionic Liquids in Synthesis; Wasserscheid, P., Welton, T., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 57–174. ISBN 978-3-527-62119-4. [Google Scholar]
- Pena-Pereira, F.; Namieśnik, J. Ionic liquids and deep eutectic mixtures: Sustainable solvents for extraction processes. Chem. Sus. Chem. 2014, 7, 1784–1800. [Google Scholar] [CrossRef]
- Xu, L.; Choi, E.-Y.; Kwon, Y.-U. Ionothermal Syntheses of Six Three-Dimensional Zinc Metal−Organic Frameworks with 1-Alkyl-3-methylimidazolium Bromide Ionic Liquids as Solvents. Inorg. Chem. 2007, 46, 10670–10680. [Google Scholar] [CrossRef]
- Xu, L.; Choi, E.-Y.; Kwon, Y.-U. Combination Effects of Cation and Anion of Ionic Liquids on the Cadmium Metal−Organic Frameworks in Ionothermal Systems. Inorg. Chem. 2008, 47, 1907–1909. [Google Scholar] [CrossRef]
- Mondal, S.S.; Bhunia, A.; Demeshko, S.; Kelling, A.; Schilde, U.; Janiak, C.; Holdt, H.-J. Synthesis of a Co (II)–imidazolate framework from an anionic linker precursor: Gas-sorption and magnetic properties. CrystEngComm 2014, 16, 39–42. [Google Scholar] [CrossRef]
- Zhou, W.-W.; Zhao, W.; Wei, B.; Du, J.-M.; Chen, Y.-H.; Tong, Y. Crystal structure of bis(µ3-hydroxy)bis(isonicotinato-κO:O’)(oxalato)- biscadmium(II)zinc(II), Cd2Zn(C2O4)(OH)2(C6NO2H4)2. Z. Kristall. 2014, 226, 611–612. [Google Scholar]
- Saha, R.; Sekar, G. Selective oxidation of alkylarenes to aromatic acids/ketone in water by using reusable binaphthyl stabilized Pt nanoparticles (Pt-BNP) as catalyst. Appl. Catal. B Environ. 2019, 250, 325–336. [Google Scholar] [CrossRef]
- Khan, I.U.; Sharif, S.; Sahin, O. Seven-, eight-, and ten-coordinated cerium(III) with highly connective pyridine-2,4,6-tricarboxylate, oxalate, and glycine ligands. J. Coord. Chem. 2013, 66, 3113–3125. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. Shape: Program. for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; Departament de Química Física, Departament de Química Inorgànica, and Institut de Química Teòrica i Computacional—Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Casanova, D.; Alemany, P.; Bofill, J.M.; Alvarez, S. Shape and Symmetry of Heptacoordinate Transition-Metal Complexes: Structural Trends. Chem. Eur. J. 2003, 9, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Ok, K.M.; Halasyamani, P.S.; Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. Distortions in Octahedrally Coordinated d0 Transition Metal Oxides: A Continuous Symmetry Measures Approach. Chem. Mater. 2006, 18, 3176–3183. [Google Scholar] [CrossRef]
- Alvarez, S.; Avnir, D.; Llunel, M.; Pinsky, M. Continuous symmetry maps and shape classification. The case of six-coordinated metal compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Liu, C.-M.; Xiong, R.-G.; You, X.-Z.; Chen, W.; Aleksa, V. A Two-Dimensional Square Network Inclusion Compound Incorporating Guest Molecules Through Both Hydrogen Bonding and Nonionic Electrostatic Attraction. Crystal Structure of [Cd(4,4′-bpy)2(H2O)2]-(ClO4)2.1.5(4,4′-bpy).(C6H4NO3Cl).H2O. Acta Chem. Scand. 1998, 52, 1353–1358. [Google Scholar] [CrossRef]
- Jiang, J.-J.; Liu, Y.-R.; Yang, R.; Pan, M.; Cao, R.; Su, C.-Y. The interplay of coordinative and hydrogen-bonding in directing the [M(4,4′-bpy)2(H2O)2] square-grid networks: Formation of 3D porous framework [Cd(4,4′-bpy)2(H2O)2](ClO4)2(4,4′-bpy)(CH3OH)2. CrystEngComm 2008, 10, 1147–1153. [Google Scholar] [CrossRef]
- Huang, S.D.; Lewandowski, B.J.; Liu, C.; Shan, Y. [Cd(4,4′-bipy)2(NO3)2](2-nitroaniline)2, a novel two-dimensional lattice inclusion compound. Acta Crystallogr. Sect. C 1999, 55, 2016–2018. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Liu, C.-M.; Zuo, J.-L.; You, X.-Z. Guest-induced dimension change. A novel network intercalation complex: {[Cd(4,4′-bipy)2(H2O)2](CF3SO3)2(4,4′-bipy)(H2O)2(C7H8N2O3)2}∞. Inorg. Chem. Commun. 1999, 2, 292–297. [Google Scholar] [CrossRef]
- Huang, S.D.; Xiong, R.-G. Molecular recognition of organic chromosphores by coordination polymers: Design and construction of nonlinear optical supramolecular assemblies. Polyhedron 1997, 16, 3929–3939. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.-Q. A new supramolecular coordination polymer constructed by flexible and rigid organic coligands. Synth. React. Inorg. Met.-Org. Nano-Metal. 2013, 43, 861–863. [Google Scholar] [CrossRef]
- Liu, C.-M.; Xiong, R.-G.; You, X.-Z.; Chen, W.; Lo, K.-M. Molecular recognition of an organic molecule through a two dimensional square network inclusion complex. Synthesis and crystal structure of [Cd(4,4′-Bpy)2(H2O)2] (BF4)2 · 2(4,4′-Bpy) · (C6H6N2O2) · 2H2O. J. Coord. Chem. 1998, 46, 211–220. [Google Scholar] [CrossRef]
- Fujita, M.; Kwon, Y.J.; Washizu, S.; Ogura, K. Preparation, clathration ability, and catalysis of a two-dimensional square network material composed of Cadmium(II) and 4,4′-Bipyridine. J. Am. Chem. Soc. 1994, 116, 1151–1152. [Google Scholar] [CrossRef]
- Hu, C.; Li, Q.; Englert, U. Structural trends in one and two dimensional coordination polymers of cadmium(II) with halide bridges and pyridine-type ligands. CrystEngComm 2003, 5, 519–529. [Google Scholar] [CrossRef]
- Zhang, J. Room-temperature compressibilities of MnO and CdO: Further examination of the role of cation type in bulk modulus systematics. Phys. Chem. Min. 1999, 26, 644–648. [Google Scholar] [CrossRef]
- Yinghua, W. Lorentz–polarization factor for correction of diffraction-line profiles. J. Appl. Crystallogr. 1987, 20, 258–259. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Han, T. International Tables for X-ray Crystallography; Kynoch Press: Birmingham, UK, 1973; Volume 4. [Google Scholar]
Sample Availability: Samples of the compound 2 are available from the authors. |






| 1 | 2 | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C14H8Cd3F2N2O8 | C10H8CdN4O6 |
| Mr | 707.42 | 392.6 |
| Crystal system, space group | Monoclinic, P21/n | Triclinic, P-1 |
| a, b, c (Å) | 10.4405 (4),7.6000(2), 12.2919(5) | 7.842(1), 9.314(1), 10.183(2) |
| α, β, γ (°) | 90, 113.019 (5), 90 | 72.72(1), 68.97(1), 69.54(1) |
| V (Å3) | 897.67 (6) | 637.8 (2) |
| Z | 2 | 2 |
| Dx (g cm−3) | 2.617 | 2.047 |
| F(000) | 664 | 384 |
| μ (mm−1) | 3.59 | 14.10 |
| Crystal size (mm) | 0.33 × 0.10 × 0.05 | 0.18 × 0.07 × 0.06 |
| Data collection | ||
| Radiation type (λ) | Mo Kα (0.71073 Å) | Cu Kα (1.54184 Å) |
| Temperature (K) | 100 | 150 |
| θ range (˚) | 3.2–27.2 | 4.7–74.3 |
| h, k, l ranges | −13 ≤ h ≤ 12, −9 ≤ k ≤ 9, −15 ≤ l ≤ 15 | −9 ≤ h ≤ 9, −11 ≤ k ≤ 10, −12 ≤ l ≤ 12 |
| Tmin, Tmax | 0.542, 0.854 | 0.397, 1 |
| No. of meas. refl. (Rint) | 5712 (0.052) | 2547 (0.057) |
| No. of independent and observed [I > 2σ(I)] refl. | 1846, 1546 | 2547, 2200 |
| (sin θ/λ)max (Å−1) | 0.643 | 0.626 |
| Refinement | ||
| R[F2 > 2σ(F2)], wR(F2), S | 0.034, 0.062, 1.05 | 0.091, 0.265, 1.12 |
| No. of reflections | 1846 | 2547 |
| No. of parameters | 145 | 191 |
| Largest diff. peak and hole (e Å−3) | 0.76 and -0.82 | 4.80and -2.12 |
| BASF | - | 0.493 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
PerezF, I.; S. Larrea, E.; Bazán, B.; Barandika, G.; Urtiaga, M.K.; Arriortua, M.I. Ionothermal Synthesis of Cadmium Coordination Polymers: Ionic Liquid Effects on the Synthesis, Structural, and Thermal Characterization. Molecules 2019, 24, 4059. https://doi.org/10.3390/molecules24224059
PerezF I, S. Larrea E, Bazán B, Barandika G, Urtiaga MK, Arriortua MI. Ionothermal Synthesis of Cadmium Coordination Polymers: Ionic Liquid Effects on the Synthesis, Structural, and Thermal Characterization. Molecules. 2019; 24(22):4059. https://doi.org/10.3390/molecules24224059
Chicago/Turabian StylePerezF, Iñigo, Edurne S. Larrea, Begoña Bazán, Gotzone Barandika, M. Karmele Urtiaga, and Maria I. Arriortua. 2019. "Ionothermal Synthesis of Cadmium Coordination Polymers: Ionic Liquid Effects on the Synthesis, Structural, and Thermal Characterization" Molecules 24, no. 22: 4059. https://doi.org/10.3390/molecules24224059