Interfacial Behavior of Oligo(Ethylene Glycol) Dendrons Spread Alone and in Combination with a Phospholipid as Langmuir Monolayers at the Air/Water Interface
Abstract
:1. Introduction
2. Results
2.1. Synthesis of OEG Dendrons
2.2. Langmuir Monolayers of OEG Dendrons Carrying a t-Bu Group, an Alkyl, or a Partially Fluorinated Chain
2.2.1. Isotherm Characteristics
2.2.2. Isotherm Reversibility
2.2.3. Brewster Angle Microscopy and Atomic Force Microscopy
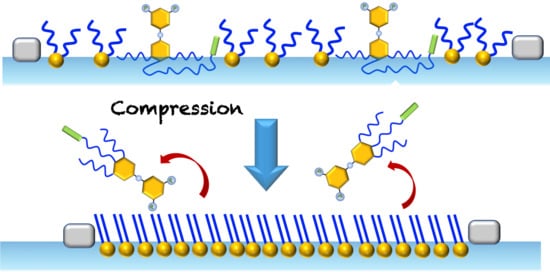
2.3. Mixed Langmuir Monolayers of Phospholipid and OEG Dendrons
2.3.1. Characteristics of the Compression Isotherms
2.3.2. Isotherm Reversibility
2.3.3. Brewster Angle Microscopy and Atomic Force Microscopy
3. Conclusions and Perspectives
4. Materials and Methods
4.1. Materials
4.2. Synthesis of the Dendrons
4.3. Langmuir Monolayers
4.4. Atomic Force Microscopy (AFM)
4.5. Brewster Angle Microscopy (BAM)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About dendrimers: Structure, physical properties, and applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef]
- Grayson, S.M.; Frechet, J.M.J. Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev. 2001, 101, 3819–3867. [Google Scholar] [CrossRef]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Int. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef]
- Rosen, B.M.; Wilson, D.A.; Wilson, C.J.; Peterca, M.; Won, B.C.; Huang, C.H.; Lipski, L.R.; Zeng, X.B.; Ungar, G.; Heiney, P.A.; et al. Predicting the structure of supramolecular dendrimers via the analysis of libraries of AB3 and constitutional isomeric AB2 biphenylpropyl ether self-assembling dendrons. J. Am. Chem. Soc. 2009, 131, 17500–17521. [Google Scholar] [CrossRef]
- Leshchiner, I.; Boiko, N.; Kumar, J.; Richardson, R.M.; Muzafarov, A.; Shibaev, V. Synthesis and physical behavior of amphiphilic dendrimers with layered organization of hydrophilic and hydrophobic blocks. Colloid Polym. Sci. 2012, 291, 927–936. [Google Scholar] [CrossRef]
- Kumar, A.; Tyagi, S.; Singh, R.; Tyagi, Y.K. Synthesis, characterisation and self-assembly studies of dendron-based novel non-ionic amphiphiles. New J. Chem. 2019, 43, 1025–1031. [Google Scholar] [CrossRef]
- Kampf, J.P.; Frank, C.W.; Malmstrom, E.E.; Hawker, C.J. Stability and molecular conformation of poly(benzyl ether) monodendrons with oligo(ethylene glycol) tails at the air-water interface. Langmuir 1999, 15, 227–233. [Google Scholar] [CrossRef]
- Basly, B.; Felder-Flesch, D.; Perriat, P.; Billotey, C.; Taleb, J.; Pourroy, G.; Begin-Colin, S. Dendronized iron oxide nanoparticles as contrast agents for MRI. Chem. Commun. 2010, 46, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Garofalo, A.; Parat, A.; Taleb, J.; Bonazza, P.; Jouhannaud, J.; Pourroy, G.; Voirin, E.; Billotey, C.; Laurent, S.; et al. Validation of a dendron concept to tune colloidal stability, MRI relaxivity and bioelimination of functional nanoparticles. J. Mater. Chem. B 2015, 3, 1484−1494. [Google Scholar] [CrossRef]
- Pasut, G.; Paolino, D.; Celia, C.; Mero, A.; Joseph, A.S.; Wolfram, J.; Cosco, D.; Schiavon, O.; Shen, H.; Fresta, M. Polyethylene glycol (PEG)-dendron phospholipids as innovative constructs for the preparation of super stealth liposomes for anticancer therapy. J. Control. Release 2015, 199, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Z.; Xu, S.L.; Deng, G.J.; Wu, P.; Fan, Q.H.; Wang, C.; Wan, L.J.; Bai, C.L. Langmuir film behaviors of dendrons at water-air interface. Chem. Phys. Lett. 2003, 370, 542–547. [Google Scholar] [CrossRef]
- Giner, I.; Haro, M.; Gascon, I.; del Barrio, J.; Lopez, M.C. Air-water interfacial behavior of linear-dendritic block copolymers containing PEG and azobenzene chromophores. J. Colloid Interface Sci. 2011, 359, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Shin, K. Dendrimers at the air-water interface: Surface dynamics and molecular ordering. Int. J. Nanotechnol. 2006, 3, 353–371. [Google Scholar] [CrossRef]
- Park, J.Y.; Advincula, R.C. Nanostructuring polymers, colloids, and nanomaterials at the air–water interface through Langmuir and Langmuir–Blodgett techniques. Soft Matter 2011, 7, 9829. [Google Scholar] [CrossRef]
- Genson, K.L.; Vaknin, D.; Villacencio, O.; McGrath, D.V.; Tsukruk, V.V. Microstructure of amphiphilic monodendrons at the air-water interface. J. Phys. Chem. B 2002, 106, 11277–11284. [Google Scholar] [CrossRef]
- Redon, R.; Carreon-Castro, M.P.; Mendoza-Martinez, F.J. Langmuir-blodgett films of supported polyester dendrimers. ISRN Org. Chem. 2012, 906839. [Google Scholar] [CrossRef]
- Su, A.H.; Tan, S.S.; Thapa, P.; Flanders, B.N.; Ford, W.T. Highly ordered Langmuir-Blodgett films of amphiphilic poly(propylene imine) dendrimers. J. Phys. Chem. C 2007, 111, 4695–4701. [Google Scholar] [CrossRef]
- Qin, L.; Duan, P.-F.; Liu, M.-H. Interfacial assembly and host–guest interaction of anthracene-conjugated l-glutamate dendron with cyclodextrin at the air/water interface. Chin. Chem. Lett. 2014, 25, 487–490. [Google Scholar] [CrossRef]
- Felipe, M.J.; Llore, N.E.; Pernites, R.B.; Nguyen, T.; Ponnapati, R.; Advincula, R.C. Interfacial behavior of OEG-linear dendron monolayers: Aggregation, nanostructuring, and electropolymerizability. Langmuir 2011, 27, 9327–9336. [Google Scholar] [CrossRef]
- Degen, P.; Wyszogrodzka, M.; Strotges, C. Film formation of nonionic dendritic amphiphiles at the water surface. Langmuir 2012, 28, 12438–12442. [Google Scholar] [CrossRef]
- Degen, P.; Wieland, D.C.; Strotges, C. Mixed layers of nonionic dendritic amphiphiles and DPPC at the water surface. Langmuir 2015, 31, 11851–11857. [Google Scholar] [CrossRef]
- Felder-Flesch, D. Dendrimers in Nanomedecine; Pan Stanford Publishing Pte. Ltd.: Singapore, 2016. [Google Scholar]
- Shi, D.; Wallyn, J.; Nguyen, D.-V.; Perton, F.; Felder-Flesch, D.; Bégin-Colin, S.; Maaloum, M.; Krafft, M.P. Microbubbles decorated with dendronized magnetic nanoparticles for biomedical imaging. Effective stabilization via fluorous interactions. Beilstein J. Nanotechnol. 2019, 10, 2103–2115. [Google Scholar] [CrossRef]
- Garofalo, A.; Parat, A.; Bordeianu, C.; Ghobril, C.; Kueny-Stotz, M.; Walter, A.; Jouhannaud, J.; Begin-Colin, S.; Felder-Flesch, D. Efficient synthesis of small-sized phosphonated dendrons: Potential organic coatings of iron oxide nanoparticles. New J. Chem. 2014, 38, 5226–5239. [Google Scholar] [CrossRef]
- Silva, A.M.G.d.; Filipe, E.J.M.; d’Oliveira, J.M.R.; Martinho, J.M.G. Interfacial behavior of poly(styrene)-poly(ethylene oxide) diblock copolymer monolayers at the air-water interface. Hydrophilic block chain length and temperature influence. Langmuir 1996, 12, 6547–6553. [Google Scholar] [CrossRef]
- Matmour, R.; Joncheray, T.J.; Gnanou, Y.; Duran, R.S. Two-dimensional polymeric nanomaterials through cross-linking of polybutadiene-b-poly(ethylene oxide) monolayers at the air/water interface. Langmuir 2007, 23, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Prasitnok, K.; Wilson, M.R. A coarse-grained model for polyethylene glycol in bulk water and at a water/air interface. Phys. Chem. Chem. Phys. 2013, 15, 17093–17104. [Google Scholar] [CrossRef]
- Schöne, A.-C.; Roch, T.; Schulz, B.; Lendlein, A. Evaluating polymeric biomaterial-environment interfaces by Langmuir monolayer techniques. Interface 2017, 14, 20161028. [Google Scholar] [CrossRef]
- Kuzmenka, D.J.; Granick, S. Collapse of poly(ethylene oxide) monolayers. Macromolecules 1988, 21, 779–782. [Google Scholar] [CrossRef]
- Bijsterbosch, H.D.; de Haan, V.O.; de Graaf, A.W.; Mellema, M.; Leermakers, F.A.M.; Cohen Stuart, M.A.; van Well, A.A. Tethered adsorbing chains: Neutron reflectivity and surface pressure of spread diblock copolymer monolayers. Langmuir 1995, 11, 4467–4473. [Google Scholar] [CrossRef]
- Fauré, M.C.; Bassereau, P.; Carignano, M.A.; Szleifer, I.; Gallot, Y.; Andelman, D. Monolayers of diblock copolymer at the air-water interface: The attractive monomer-surface case. Eur. Phys. J. B 1998, 3, 365–375. [Google Scholar]
- Deschênes, L.; Saint-Germain, F.; Lyklema, J. Langmuir monolayers of non-ionic polymers: Equilibrium or metastability? Case study of PEO and its PPO–PEO diblock copolymers. J. Colloid Interface Sci. 2015, 449, 494–505. [Google Scholar]
- Krafft, M.P.; Riess, J.G. Chemistry, physical chemistry and uses of molecular fluorocarbon-hydrocarbon diblocks, triblocks and related compounds—Unique apolar components for self-assembled colloid and interface engineering. Chem. Rev. 2009, 109, 1714–1792. [Google Scholar] [CrossRef]
- Krafft, M.P. Large organized surface domains self-assembled from non-polar amphiphiles. Acc. Chem. Res. 2012, 45, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Smart, B.E. Characteristics of C-F systems. In Organofluorine Chemistry: Principles and Commercial Applications; Banks, R.E., Smart, B.E., Tatlow, J.C., Eds.; Plenum Press: New York, NY, USA, 1994; Chap. 3; pp. 57–88. [Google Scholar]
- Krafft, M.P.; Riess, J.G. Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability-Part one. Chemosphere 2015, 129, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.K.; Papadopoulou, V.; Dayton, P.A. Imaging with ultrasound contrast agents: Current status and future. Abdom. Radiol. 2018, 43, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Sirsi, S.; Borden, M. Microbubble compositions, properties and biomedical applications. Bubble Sci. Eng. Technol. 2009, 1, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Schutt, E.S.; Klein, D.H.; Mattrey, R.M.; Riess, J.G. Injectable microbubbles as contrast agents for diagnostic ultrasound imaging: The key role of perfluorochemicals. Angew. Chem. Int. Ed. 2003, 42, 3218–3235. [Google Scholar] [CrossRef]
- Shibata, O.; Krafft, M.P. Mixed Langmuir monolayers made from single-chain perfluoroalkylated amphiphiles. Langmuir 2000, 16, 10281–10286. [Google Scholar] [CrossRef]
- Krafft, M.P.; Goldmann, M. Monolayers made from fluorinated amphiphiles. Curr. Opin. Colloid Interf. Sci. 2003, 8, 243–250. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |










| Dendrons | A0 (Å2) ± 50 Å2 | πplateau (mN m−1) ± 1 mN m−1 |
|---|---|---|
| t-BuOEG4Den | 750 | 6.0 |
| t-BuOEG6Den | 850 | 6.5 |
| t-BuOEG8Den | 900 | 9.6 |
| C6H13OEG4Den | 700 | 7.2 |
| C6H13OEG6Den | 720 | 9.9 |
| C6H13OEG8Den | 850 | 9.1 |
| C2F5C4H8OEG8Den | 780 | 13.1 |
| C8H17OEG8Den | 470 | 18.3 |
| C4F9C4H8OEG8Den | 580 | 17.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, D.; Nguyen, D.-V.; Maaloum, M.; Gallani, J.-L.; Felder-Flesch, D.; Krafft, M.P. Interfacial Behavior of Oligo(Ethylene Glycol) Dendrons Spread Alone and in Combination with a Phospholipid as Langmuir Monolayers at the Air/Water Interface. Molecules 2019, 24, 4114. https://doi.org/10.3390/molecules24224114
Shi D, Nguyen D-V, Maaloum M, Gallani J-L, Felder-Flesch D, Krafft MP. Interfacial Behavior of Oligo(Ethylene Glycol) Dendrons Spread Alone and in Combination with a Phospholipid as Langmuir Monolayers at the Air/Water Interface. Molecules. 2019; 24(22):4114. https://doi.org/10.3390/molecules24224114
Chicago/Turabian StyleShi, Da, Dinh-Vu Nguyen, Mounir Maaloum, Jean-Louis Gallani, Delphine Felder-Flesch, and Marie Pierre Krafft. 2019. "Interfacial Behavior of Oligo(Ethylene Glycol) Dendrons Spread Alone and in Combination with a Phospholipid as Langmuir Monolayers at the Air/Water Interface" Molecules 24, no. 22: 4114. https://doi.org/10.3390/molecules24224114