Electrochemical Hydrogen Evolution over Hydrothermally Synthesized Re-Doped MoS2 Flower-Like Microspheres
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Catalyst
2.1.1. Scanning Electron Microscopy Analysis
2.1.2. X-ray Diffraction Analysis
2.1.3. Raman Spectroscopy Analysis
2.1.4. Scanning Transmission Electron Microscopy Analysis
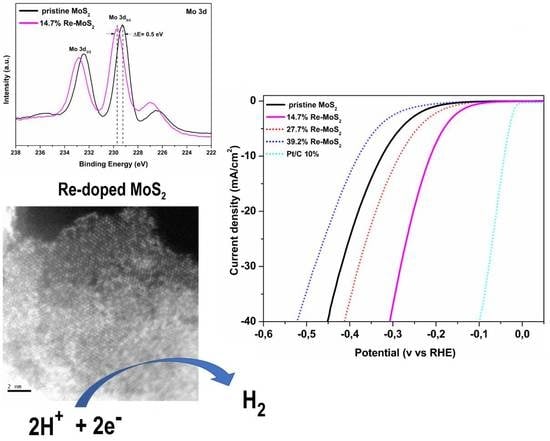
2.1.5. X-Ray Photoelectron Spectroscopy
2.2. Hydrogen Evolution Reaction (HER) Performance of Pristine MoS2 and Re-doped MoS2 Samples
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Re-Doped MoS2 and Pristine MoS2
3.3. Characterization Techniques
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Voiry, D.; Yang, J.; Chhowalla, M. Recent strategies for improving the catalytic activity of 2D TMD nanosheets toward the hydrogen evolution reaction. Adv. Mater. 2016, 28, 6197–6206. [Google Scholar] [CrossRef] [PubMed]
- Vesborg, P.C.K.; Seger, B.; Chorkendorff, I.B. Recent development in hydrogen evolution reaction catalysts and their practical implementation. J. Phys. Chem. Lett. 2015, 6, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Chen, W.; Wang, X. A Review of Phosphide-Based Materials for Electrocatalytic Hydrogen Evolution. Adv. Energy Mater. 2015, 5, 1500985. [Google Scholar] [CrossRef]
- Rajamathi, C.R.; Gupta, U.; Kumar, N.; Yang, H.; Sun, Y.; Süß, V.; Shekhar, C.; Schmidt, M.; Blumtritt, H.; Werner, P.; et al. Weyl Semimetals as Hydrogen Evolution Catalysts. Adv. Mater. 2017, 29, 1606202. [Google Scholar] [CrossRef]
- Rasaki, S.A.; Zhang, B.; Anbalgam, K.; Thomas, T.; Yang, M. Synthesis and application of nano-structured metal nitrides and carbides: A review. Prog. Solid State Chem. 2018, 50, 1–15. [Google Scholar] [CrossRef]
- Tsai, C.; Chan, K.; Nørskov, J.K.; Abild-Pedersen, F. Theoretical insights into the hydrogen evolution activity of layered transition metal dichalcogenides. Surf. Sci. 2015, 640, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Hai, X.; Chang, K.; Pang, H.; Li, M.; Li, P.; Liu, H.; Shi, L.; Ye, J. Engineering the edges of MoS2 (WS2) crystals for direct exfoliation into monolayers in polar micromolecular solvents. J. Am. Chem. Soc. 2016, 138, 14962–14969. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [Green Version]
- Chung, D.Y.; Park, S.-K.; Chung, Y.-H.; Yu, S.-H.; Lim, D.-H.; Jung, N.; Ham, H.C.; Park, H.-Y.; Piao, Y.; Yoo, S.J.; et al. Edge-exposed MoS2 nano-assembled structures as efficient electrocatalysts for hydrogen evolution reaction. Nanoscale 2014, 6, 2131–2136. [Google Scholar] [CrossRef]
- Huang, X.; Leng, M.; Xiao, W.; Li, M.; Ding, J.; Tan, T.L.; Lee, W.S.V.; Xue, J. Activating basal planes and Sterminated edges of MoS2 toward more efficient hydrogen evolution. Adv. Funct. Mater. 2017, 27, 1604943. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R.; Liu, S.; Zhuang, X.; Feng, X. Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overallwatersplitting activity. Angew. Chemie Int. Ed. 2016, 55, 6702–6707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Zhu, J.; Zhang, J.; Tao, K.; Gao, D.; Xi, P. Active basal plane catalytic activity and conductivity in Zn doped MoS2 nanosheets for efficient hydrogen evolution. Electrochim. Acta 2018, 260, 24–30. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, F.; Zhang, S.; Liang, Y.; Wang, R. Engineering MoS2 Basal Planes for Hydrogen Evolution via Synergistic Ruthenium Doping and Nanocarbon Hybridization. Adv. Sci. 2019, 6, 1900090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolar, S.; Shit, S.; Kumar, J.S.; Murmu, N.C.; Ganesh, R.S.; Inokawa, H.; Kuila, T. Optimization of active surface area of flower like MoS2 using V-doping towards enhanced hydrogen evolution reaction in acidic and basic medium. Appl. Catal. B Environ. 2019, 254, 432–442. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, Y.; Li, S.; He, B.; Liu, H.; Zeng, X.; Zhang, J.; Wang, G. P doped MoS2 nanoplates embedded in nitrogen doped carbon nanofibers as an efficient catalyst for hydrogen evolution reaction. J. Colloid Interface Sci. 2019, 547, 291–298. [Google Scholar] [CrossRef]
- Yang, S.; Gong, Y.; Manchanda, P.; Zhang, Y.; Ye, G.; Chen, S.; Song, L.; Pantelides, S.T.; Ajayan, P.M.; Chisholm, M.F. Rhenium Doped and Stabilized MoS2 Atomic Layers with Basal Plane Catalytic Activity. Adv. Mater. 2018, 30, 1803477. [Google Scholar] [CrossRef]
- Chhetri, M.; Gupta, U.; Yadgarov, L.; Rosentsveig, R.; Tenne, R.; Rao, C.N.R. Beneficial effect of Re doping on the electrochemical HER activity of MoS2 fullerenes. Dalt. Trans. 2015, 44, 16399–16404. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Liu, P.; Liu, Y.; Gao, D.; Xue, D.; Ding, J. Re doping induced 2H-1T phase transformation and ferromagnetism in MoS2 nanosheets. Appl. Phys. Lett. 2018, 113, 13101. [Google Scholar] [CrossRef]
- Dalmatova, S.A.; Fedorenko, A.D.; Mazalov, L.N.; Asanov, I.P.; Ledneva, A.Y.; Tarasenko, M.S.; Enyashin, A.N.; Zaikovskii, V.I.; Fedorov, V.E. XPS experimental and DFT investigations on solid solutions of Mo1−xRexS2 (0 < x < 0.20). Nanoscale 2018, 10, 10232–10240. [Google Scholar]
- Chhetri, M.; Gupta, U.; Yadgarov, L.; Rosentsveig, R.; Tenne, R.; Rao, C.N.R. Effects of pand ntype Doping in Inorganic Fullerene MoS2 on the Hydrogen Evolution Reaction. ChemElectroChem 2016, 3, 1937–1943. [Google Scholar] [CrossRef]
- Ji, L.; Yan, P.; Zhu, C.; Ma, C.; Wu, W.; Wei, C.; Shen, Y.; Chu, S.; Wang, J.; Du, Y.; et al. One-pot synthesis of porous 1T-phase MoS2 integrated with single-atom Cu doping for enhancing electrocatalytic hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 251, 87–93. [Google Scholar] [CrossRef]
- Vasu, K.; Meiron, O.E.; Enyashin, A.N.; Bar-Ziv, R.; Bar-Sadan, M. Effect of Ru Doping on the Properties of MoSe2 Nanoflowers. J. Phys. Chem. C 2019, 123, 1987–1994. [Google Scholar] [CrossRef]
- Al-Dulaimi, N.; Lewis, E.A.; Savjani, N.; McNaughter, P.D.; Haigh, S.J.; Malik, M.A.; Lewis, D.J.; O’Brien, P. The influence of precursor on rhenium incorporation into Re-doped MoS2 (Mo1−xRexS2) thin films by aerosol-assisted chemical vapour deposition (AACVD). J. Mater. Chem. C 2017, 5, 9044–9052. [Google Scholar] [CrossRef]
- Li, Y.; Yin, K.; Wang, L.; Lu, X.; Zhang, Y.; Liu, Y.; Yan, D.; Song, Y.; Luo, S. Engineering MoS2 nanomesh with holes and lattice defects for highly active hydrogen evolution reaction. Appl. Catal. B Environ. 2018, 239, 537–544. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, H.; Zheng, Y.; Hu, Y.; MacLennan, A. Understanding of the effect of synthesis temperature on the crystallization and activity of nano-MoS2 catalyst. Appl. Catal. B Environ. 2015, 165, 537–546. [Google Scholar] [CrossRef]
- Blanco, É.; Afanasiev, P.; Berhault, G.; Uzio, D.; Loridant, S. Resonance Raman spectroscopy as a probe of the crystallite size of MoS2 nanoparticles. Comptes. Rendus. Chim. 2016, 19, 1310–1314. [Google Scholar] [CrossRef]
- Afanasiev, P. Topotactic synthesis of size-tuned MoS2 inorganic fullerenes that allows revealing particular catalytic properties of curved basal planes. Appl. Catal. B Environ. 2018, 227, 44–53. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, G.; Luo, R.; Johnson, I.; Kashani, H.; Chen, M. Chemical doping induced zone-edge phonon renormalization in single-layer MoS2. Phys. Rev. B 2019, 100, 85401. [Google Scholar] [CrossRef]
- Tongay, S.; Sahin, H.; Ko, C.; Luce, A.; Fan, W.; Liu, K.; Zhou, J.; Huang, Y.-S.; Ho, C.-H.; Yan, J.; et al. Monolayer behaviour in bulk ReS2 due to electronic and vibrational decoupling. Nat. Commun. 2014, 5, 3252. [Google Scholar] [CrossRef] [Green Version]
- Benavente, E.; Aliaga, J.A.; Barraza, P.; Araya, J.F.; Farías, M.H.; González, G.; Alonso-Núñez, G. Melamine-assisted synthesis of nitrogen-doped ReS2 nanosheets/carbon composites. Mater. Lett. 2019, 243, 42–45. [Google Scholar] [CrossRef]
- Ekspong, J.; Sandström, R.; Rajukumar, L.P.; Terrones, M.; Wågberg, T.; Gracia-Espino, E. Stable Sulfur-Intercalated 1T′ MoS2 on Graphitic Nanoribbons as Hydrogen Evolution Electrocatalyst. Adv. Funct. Mater. 2018, 28, 1802744. [Google Scholar] [CrossRef]
- Mignuzzi, S.; Pollard, A.J.; Bonini, N.; Brennan, B.; Gilmore, I.S.; Pimenta, M.A.; Richards, D.; Roy, D. Effect of disorder on Raman scattering of single-layer MoS2. Phys. Rev. B 2015, 91, 195411. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Kim, Y.D.; Liang, L.; Idrobo, J.C.; Chow, P.; Tan, J.; Li, B.; Li, L.; Sumpter, B.G.; Lu, T.-M.; et al. Transition-Metal Substitution Doping in Synthetic Atomically Thin Semiconductors. Adv. Mater. 2016, 28, 9735–9743. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Dong, W.F.; Li, B.L.; Deng, Y.H.; Wang, X.H.; Zhang, X.F.; Luo, H.Q.; Li, N.B. Cobalt incorporated MoS2 hollow structure with rich out-of-plane edges for efficient hydrogen production. Electrochim. Acta 2018, 276, 81–91. [Google Scholar] [CrossRef]
- Luo, Z.; Ouyang, Y.; Zhang, H.; Xiao, M.; Ge, J.; Jiang, Z.; Wang, J.; Tang, D.; Cao, X.; Liu, C. Chemically activating MoS2 via spontaneous atomic palladium interfacial doping towards efficient hydrogen evolution. Nat. Commun. 2018, 9, 2120. [Google Scholar] [CrossRef] [Green Version]
- Bian, X.; Zhu, J.; Liao, L.; Scanlon, M.D.; Ge, P.; Ji, C.; Girault, H.H.; Liu, B. Nanocomposite of MoS2 on ordered mesoporous carbon nanospheres: A highly active catalyst for electrochemical hydrogen evolution. Electrochem. Commun. 2012, 22, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Ning, S.; Fu, W.; Pennycook, S.J.; Loh, K.P. Differentiating Polymorphs in Molybdenum Disulfide via Electron Microscopy. Adv. Mater. 2018, 30, 1802397. [Google Scholar] [CrossRef]
- Baker, M.A.; Gilmore, R.; Lenardi, C.; Gissler, W. XPS investigation of preferential sputtering of S from MoS2 and determination of MoSx stoichiometry from Mo and S peak positions. Appl. Surf. Sci. 1999, 150, 255–262. [Google Scholar] [CrossRef]






| Sample | Mo 3d5/2 | S 2p3/2 | Re 4f7/2 | Composition |
|---|---|---|---|---|
| Pristine MoS2 | 229.3 | 161.8 | - | MoS1.64 |
| 14.7% Re-doped MoS2 | 229.7 | 162.6 | 42.4 | Mo0.85Re0.15S1.76 |
| 27.7% Re-doped MoS2 | 229.7 | 162.5 | 42.2 | Mo0.72Re0.28S1.73 |
| 39.2 % Re-doped MoS2 | 229.8, 228.8 | 162.8, 161.7 | 42.7, 41.6 | Mo0.61Re0.39S1.83 |
| Sample | Onset Potential (mV) | η10 (mV) | Tafel Slope (mVdec−1) | Rct (Ωcm2) |
|---|---|---|---|---|
| Pristine MoS2 | 203 | 326 | 102 | 32.58 |
| 14.7% Re-doped MoS2 | 123 | 210 | 78 | 7.77 |
| 27.7% Re-doped MoS2 | 164 | 285 | 97 | 17.45 |
| 39.2% Re-doped MoS2 | 244 | 379 | 132 | 157.86 |
| Pt/C 10% | 20 | 42 | 32 | - |
| Sample | (NH4)2MoO4 | (NH4)ReO4 | CH4N2S |
|---|---|---|---|
| Pristine MoS2 | 2.0 mmol | - | 6.0 mmol |
| 14.7% Re-doped MoS2 | 1.8 mmol | 0.2 mmol | 6.0 mmol |
| 27.7% Re-doped MoS2 | 1.5 mmol | 0.5 mmol | 6.0 mmol |
| 39.2% Re-doped MoS2 | 1.0 mmol | 1.0 mmol | 6.0 mmol |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliaga, J.; Vera, P.; Araya, J.; Ballesteros, L.; Urzúa, J.; Farías, M.; Paraguay-Delgado, F.; Alonso-Núñez, G.; González, G.; Benavente, E. Electrochemical Hydrogen Evolution over Hydrothermally Synthesized Re-Doped MoS2 Flower-Like Microspheres. Molecules 2019, 24, 4631. https://doi.org/10.3390/molecules24244631
Aliaga J, Vera P, Araya J, Ballesteros L, Urzúa J, Farías M, Paraguay-Delgado F, Alonso-Núñez G, González G, Benavente E. Electrochemical Hydrogen Evolution over Hydrothermally Synthesized Re-Doped MoS2 Flower-Like Microspheres. Molecules. 2019; 24(24):4631. https://doi.org/10.3390/molecules24244631
Chicago/Turabian StyleAliaga, Juan, Pablo Vera, Juan Araya, Luis Ballesteros, Julio Urzúa, Mario Farías, Francisco Paraguay-Delgado, Gabriel Alonso-Núñez, Guillermo González, and Eglantina Benavente. 2019. "Electrochemical Hydrogen Evolution over Hydrothermally Synthesized Re-Doped MoS2 Flower-Like Microspheres" Molecules 24, no. 24: 4631. https://doi.org/10.3390/molecules24244631