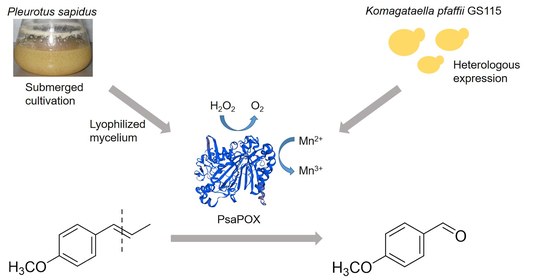
A DyP-Type Peroxidase of Pleurotus sapidus with Alkene Cleaving Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification and Identification of the Alkene Cleavage Activity
2.2. Amplification and Expression of PsaPOX
2.3. Production and Purification of the Recombinant PsaPOX
2.4. Biochemical Characterization of PsaPOX
2.5. Alkene Cleavage Activity of PsaPOX
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Cultivation of P. sapidus
3.3. Purification Strategy
3.4. Gel Electrophoresis
3.5. Isoelectric Focussing
3.6. Peptide Mass Fingerprinting
3.7. cDNA Synthesis and Gene Amplification
3.8. Heterologous Expression of PsaPOX in Komagataella pfaffii
3.9. His-Tag Purification of Recombinant PsaPOX
3.10. Biotransformation
3.11. Enzyme Activities
3.12. Biochemical Characterization of PsaPOX
3.13. Alkene Cleavage Activity of PsaPOX
3.14. Detection of Hydrogen Peroxide
3.15. Sequence Accession Numbers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fahlbusch, K.-G.; Hammerschmidt, F.-J.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. Flavors and Fragrances. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 73–140. [Google Scholar]
- Spannring, P.; Bruijnincx, P.C.A.; Weckhuysen, B.M.; Klein Gebbink, R.J.M. Transition metal-catalyzed oxidative double bond cleavage of simple and bio-derived alkenes and unsaturated fatty acids. Catal. Sci. Technol. 2014, 4, 2182–2209. [Google Scholar] [CrossRef]
- Rajagopalan, A.; Lara, M.; Kroutil, W. Oxidative Alkene Cleavage by Chemical and Enzymatic Methods. Adv. Synth. Catal. 2013, 355, 3321–3335. [Google Scholar] [CrossRef]
- Criegee, R. Mechanism of Ozonolysis. Angew. Chemie Int. Ed. Engl. 1975, 14, 745–752. [Google Scholar] [CrossRef]
- Schrader, J.; Etschmann, M.M.W.; Sell, D.; Hilmer, J.-M.; Rabenhorst, J. Applied biocatalysis for the synthesis of natural flavour compounds – current industrial processes and future prospects. Biotechnol. Lett. 2004, 26, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, A.; Schober, M.; Emmerstorfer, A.; Hammerer, L.; Migglautsch, A.; Seisser, B.; Glueck, S.M.; Niehaus, F.; Eck, J.; Pichler, H.; et al. Enzymatic Aerobic Alkene Cleavage Catalyzed by a Mn3+-Dependent Proteinase A Homologue. ChemBioChem 2013, 14, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Ryu, J.-Y.; Kanaly, R.A.; Hur, H.-G. Isolation of a Gene Responsible for the Oxidation of trans-Anethole to para-Anisaldehyde by Pseudomonas putida JYR-1 and Its Expression in Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 5238–5246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, M.; Okada, Y.; Yoshida, T.; Nagasawa, T. Purification, characterization and gene cloning of isoeugenol-degrading enzyme from Pseudomonas putida IE27. Arch. Microbiol. 2007, 187, 511–517. [Google Scholar] [CrossRef]
- Hajnal, I.; Faber, K.; Schwab, H.; Hall, M.; Steiner, K. Oxidative Alkene Cleavage Catalysed by Manganese-Dependent Cupin TM1459 from Thermotoga maritima. Adv. Synth. Catal. 2015, 357, 3309–3316. [Google Scholar] [CrossRef]
- Tuynman, A.; Spelberg, J.L.; Kooter, I.M.; Schoemaker, H.E.; Wever, R. Enantioselective Epoxidation and Carbon–Carbon Bond Cleavage Catalyzed by Coprinus cinereus Peroxidase and Myeloperoxidase. J. Biol. Chem. 2000, 275, 3025–3030. [Google Scholar] [CrossRef] [Green Version]
- Mutti, F.G.; Lara, M.; Kroutil, M.; Kroutil, W. Ostensible Enzyme Promiscuity: Alkene Cleavage by Peroxidases. Chem. Eur. J. 2010, 16, 14142–14148. [Google Scholar] [CrossRef]
- Hofrichter, M.; Ullrich, R.; Pecyna, M.J.; Liers, C.; Lundell, T. New and classic families of secreted fungal heme peroxidases. Appl. Microbiol. Biotechnol. 2010, 87, 871–897. [Google Scholar] [CrossRef] [PubMed]
- Behrens, C.J.; Zelena, K.; Berger, R.G. Comparative Cold Shock Expression and Characterization of Fungal Dye-Decolorizing Peroxidases. Appl. Biochem. Biotechnol. 2016, 179, 1404–1417. [Google Scholar] [CrossRef] [PubMed]
- Sugano, Y. DyP-type peroxidases comprise a novel heme peroxidase family. Cell. Mol. Life Sci. 2009, 66, 1387–1403. [Google Scholar] [CrossRef] [PubMed]
- Linde, D.; Ruiz-Dueñas, F.J.; Fernández-Fueyo, E.; Guallar, V.; Hammel, K.E.; Pogni, R.; Martínez, A.T. Basidiomycete DyPs: Genomic diversity, structural–functional aspects, reaction mechanism and environmental significance. Arch. Biochem. Biophys. 2015, 574, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Strittmatter, E.; Liers, C.; Ullrich, R.; Wachter, S.; Hofrichter, M.; Plattner, D.A.; Piontek, K. First Crystal Structure of a Fungal High-redox Potential Dye-decolorizing Peroxidase. J. Biol. Chem. 2013, 288, 4095–4102. [Google Scholar] [CrossRef] [Green Version]
- Rais, D.; Zibek, S. Biotechnological and Biochemical Utilization of Lignin. In Biorefineries; Wagemann, K., Tippkötter, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 469–518. [Google Scholar]
- Salvachua, D.; Prieto, A.; Martinez, A.T.; Martinez, M.J. Characterization of a Novel Dye-Decolorizing Peroxidase (DyP)-Type Enzyme from Irpex lacteus and Its Application in Enzymatic Hydrolysis of Wheat Straw. Appl. Environ. Microbiol. 2013, 79, 4316–4324. [Google Scholar] [CrossRef] [Green Version]
- Lauber, C.; Schwarz, T.; Nguyen, Q.K.; Lorenz, P.; Lochnit, G.; Zorn, H. Identification, heterologous expression and characterization of a dye-decolorizing peroxidase of Pleurotus sapidus. AMB Express 2017, 7, 164. [Google Scholar] [CrossRef] [Green Version]
- Busse, N.; Wagner, D.; Kraume, M.; Czermak, P. Reaction Kinetics of Versatile Peroxidase for the Degradation of Lignin Compounds. Am. J. Biochem. Biotechnol. 2013, 9, 365–394. [Google Scholar] [CrossRef]
- Arnao, M.B.; Acosta, M.; del Rio, J.A.; Varón, R.; García-Cánovas, F. A kinetic study on the suicide inactivation of peroxidase by hydrogen peroxide. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1990, 1041, 43–47. [Google Scholar] [CrossRef]
- Dako, E.; Bernier, A.-M.; Thomas, A.; Jankowski, C.K. The Problems Associated with Enzyme Purification. In Chemical Biology; Ekinci, D., Ed.; InTech: Rijeka, Croatia, 2012; pp. 19–40. [Google Scholar]
- Schulz, K.; Nieter, A.; Scheu, A.-K.; Copa-Patiño, J.L.; Thiesing, D.; Popper, L.; Berger, R.G. A type D ferulic acid esterase from Streptomyces werraensis affects the volume of wheat dough pastries. Appl. Microbiol. Biotechnol. 2018, 102, 1269–1279. [Google Scholar] [CrossRef]
- Kolwek, J.; Behrens, C.; Linke, D.; Krings, U.; Berger, R.G. Cell-free one-pot conversion of (+)-valencene to (+)-nootkatone by a unique dye-decolorizing peroxidase combined with a laccase from Funalia trogii. J. Ind. Microbiol. Biotechnol. 2018, 45, 89–101. [Google Scholar] [CrossRef]
- Hofrichter, M. Review: Lignin conversion by manganese peroxidase (MnP). Enzyme Microb. Technol. 2002, 30, 454–466. [Google Scholar] [CrossRef]
- Nakayama, T.; Amachi, T. Fungal peroxidase: Its structure, function, and application. J. Mol. Catal. B Enzym. 1999, 6, 185–198. [Google Scholar] [CrossRef]
- Fernández-Fueyo, E.; Linde, D.; Almendral, D.; López-Lucendo, M.F.; Ruiz-Dueñas, F.J.; Martínez, A.T. Description of the first fungal dye-decolorizing peroxidase oxidizing manganese(II). Appl. Microbiol. Biotechnol. 2015, 99, 8927–8942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Fueyo, E.; Davó-Siguero, I.; Almendral, D.; Linde, D.; Baratto, M.C.; Pogni, R.; Romero, A.; Guallar, V.; Martínez, A.T. Description of a Non-Canonical Mn(II)-Oxidation Site in Peroxidases. ACS Catal. 2018, 8, 8386–8395. [Google Scholar] [CrossRef]
- Linde, D.; Pogni, R.; Cañellas, M.; Lucas, F.; Guallar, V.; Baratto, M.C.; Sinicropi, A.; Sáez-Jiménez, V.; Coscolín, C.; Romero, A.; et al. Catalytic surface radical in dye-decolorizing peroxidase: a computational, spectroscopic and site-directed mutagenesis study. Biochem. J. 2015, 466, 253–262. [Google Scholar] [CrossRef]
- Amara, S.; Perrot, T.; Navarro, D.; Deroy, A.; Benkhelfallah, A.; Chalak, A.; Daou, M.; Chevret, D.; Faulds, C.B.; Berrin, J.-G.; et al. Enzyme Activities of Two Recombinant Heme-Containing Peroxidases, Tv DyP1 and Tv VP2, Identified from the Secretome of Trametes versicolor. Appl. Environ. Microbiol. 2018, 84, e02826-17. [Google Scholar] [CrossRef] [Green Version]
- Duan, Z.; Shen, R.; Liu, B.; Yao, M.; Jia, R. Comprehensive investigation of a dye-decolorizing peroxidase and a manganese peroxidase from Irpex lacteus F17, a lignin-degrading basidiomycete. AMB Express 2018, 8, 119. [Google Scholar] [CrossRef]
- Passardi, F.; Theiler, G.; Zamocky, M.; Cosio, C.; Rouhier, N.; Teixera, F.; Margis-Pinheiro, M.; Ioannidis, V.; Penel, C.; Falquet, L.; et al. PeroxiBase: The peroxidase database. Phytochemistry 2007, 68, 1605–1611. [Google Scholar] [CrossRef]
- Colpa, D.I.; Fraaije, M.W.; van Bloois, E. DyP-type peroxidases: a promising and versatile class of enzymes. J. Ind. Microbiol. Biotechnol. 2014, 41, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Romanos, M. Advances in the use of Pichia pastoris for high-level gene expression. Curr. Opin. Biotechnol. 1995, 6, 527–533. [Google Scholar] [CrossRef]
- Ogawa, S.; Shimizu, T.; Ohki, H.; Araya, T.; Okuno, T.; Miyairi, K. Expression, purification, and analyses of glycosylation and disulfide bonds of Stereum purpureum endopolygalacturonase I in Pichia pastoris. Protein Expr. Purif. 2009, 65, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Behrens, C.J.; Linke, D.; Allister, A.B.; Zelena, K.; Berger, R.G. Variants of PpuLcc, a multi-dye decolorizing laccase from Pleurotus pulmonarius expressed in Pichia pastoris. Protein Expr. Purif. 2017, 137, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Avram, A.; Sengupta, A.; Pfromm, P.H.; Zorn, H.; Lorenz, P.; Schwarz, T.; Nguyen, K.Q.; Czermak, P. Novel DyP from the basidiomycete Pleurotus sapidus: substrate screening and kinetics. Biocatalysis 2018, 4, 1–13. [Google Scholar] [CrossRef]
- Rahmanpour, R.; Bugg, T.D.H. Characterisation of Dyp-type peroxidases from Pseudomonas fluorescens Pf-5: Oxidation of Mn(II) and polymeric lignin by Dyp1B. Arch. Biochem. Biophys. 2015, 574, 93–98. [Google Scholar] [CrossRef]
- Linke, D.; Leonhardt, R.; Eisele, N.; Petersen, L.M.; Riemer, S.; Nimtz, M.; Berger, R.G. Carotene-degrading activities from Bjerkandera adusta possess an application in detergent industries. Bioprocess Biosyst. Eng. 2015, 38, 1191–1199. [Google Scholar] [CrossRef]
- Scheibner, M.; Hülsdau, B.; Zelena, K.; Nimtz, M.; de Boer, L.; Berger, R.G.; Zorn, H. Novel peroxidases of Marasmius scorodonius degrade β-carotene. Appl. Microbiol. Biotechnol. 2008, 77, 1241–1250. [Google Scholar] [CrossRef] [Green Version]
- Zorn, H.; Langhoff, S.; Scheibner, M.; Nimtz, M.; Berger, R.G. A Peroxidase from Lepista irina Cleaves β, β-Carotene to Flavor Compounds. Biol. Chem. 2003, 384, 1049–1056. [Google Scholar] [CrossRef]
- Krings, U.; Lehnert, N.; Fraatz, M.A.; Hardebusch, B.; Zorn, H.; Berger, R.G. Autoxidation versus biotransformation of α-pinene to flavors with Pleurotus sapidus: Regioselective hydroperoxidation of α-pinene and stereoselective dehydrogenation of verbenol. J. Agric. Food Chem. 2009, 57, 9944–9950. [Google Scholar] [CrossRef]
- Linke, D.; Omarini, A.B.; Takenberg, M.; Kelle, S.; Berger, R.G. Long-Term Monokaryotic Cultures of Pleurotus ostreatus var. florida Produce High and Stable Laccase Activity Capable to Degrade ß-Carotene. Appl. Biochem. Biotechnol. 2019, 187, 894–912. [Google Scholar] [CrossRef]
- Schulz, K.; Giesler, L.; Linke, D.; Berger, R.G. A prolyl endopeptidase from Flammulina velutipes for the possible degradation of celiac disease provoking toxic peptides in cereal proteins. Process Biochem. 2018, 73, 47–55. [Google Scholar] [CrossRef]
- Nieter, A.; Haase-Aschoff, P.; Linke, D.; Nimtz, M.; Berger, R.G. A halotolerant type A feruloyl esterase from Pleurotus eryngii. Fungal Biol. 2014, 118, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Lin-Cereghino, J.; Wong, W.W.; Xiong, S.; Giang, W.; Luong, L.T.; Vu, J.; Johnson, S.D.; Lin-Cereghino, G.P. Condensed protocol for competent cell preparation and transformation of the methylotrophic yeast Pichia pastoris. Biotechniques 2005, 38, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Sygmund, C.; Gutmann, A.; Krondorfer, I.; Kujawa, M.; Glieder, A.; Pscheidt, B.; Haltrich, D.; Peterbauer, C.; Kittl, R. Simple and efficient expression of Agaricus meleagris pyranose dehydrogenase in Pichia pastoris. Appl. Microbiol. Biotechnol. 2012, 94, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Nieter, A.; Kelle, S.; Takenberg, M.; Linke, D.; Bunzel, M.; Popper, L.; Berger, R.G. Heterologous production and characterization of a chlorogenic acid esterase from Ustilago maydis with a potential use in baking. Food Chem. 2016, 209, 1–9. [Google Scholar] [CrossRef]
- Britton, H.T.S.; Robinson, R.A. CXCVIII.—Universal buffer solutions and the dissociation constant of veronal. J. Chem. Soc. 1931, 1456–1462. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrpugh, N.J.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Fink, M.; Trunk, S.; Hall, M.; Schwab, H.; Steiner, K. Engineering of TM1459 from Thermotoga maritima for increased oxidative alkene cleavage activity. Front. Microbiol. 2016, 7, 1511. [Google Scholar] [CrossRef]
Sample Availability: Samples of the pPIC9 vector containing the PsaPOX gene are available from the authors. |








| Substrate | Km (µM) | kcat (s−1) | kcat/Km (s−1 mM−1) |
|---|---|---|---|
| ABTS | 37 ± 4 | 6.8 ± 0.2 | 184 ± 5 |
| Mn2+ | 1025 ± 79 | 7.2 ± 0.1 | 7 ± 0.1 |
| RB19 | n. d. | - | - |
| RB5 | n. d. | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krahe, N.-K.; Berger, R.G.; Ersoy, F. A DyP-Type Peroxidase of Pleurotus sapidus with Alkene Cleaving Activity. Molecules 2020, 25, 1536. https://doi.org/10.3390/molecules25071536
Krahe N-K, Berger RG, Ersoy F. A DyP-Type Peroxidase of Pleurotus sapidus with Alkene Cleaving Activity. Molecules. 2020; 25(7):1536. https://doi.org/10.3390/molecules25071536
Chicago/Turabian StyleKrahe, Nina-Katharina, Ralf G. Berger, and Franziska Ersoy. 2020. "A DyP-Type Peroxidase of Pleurotus sapidus with Alkene Cleaving Activity" Molecules 25, no. 7: 1536. https://doi.org/10.3390/molecules25071536