Enhancement of the Antioxidant, Anti-Tyrosinase, and Anti-Hyaluronidase Activity of Morus alba L. Leaf Extract by Pulsed Electric Field Extraction
Abstract
: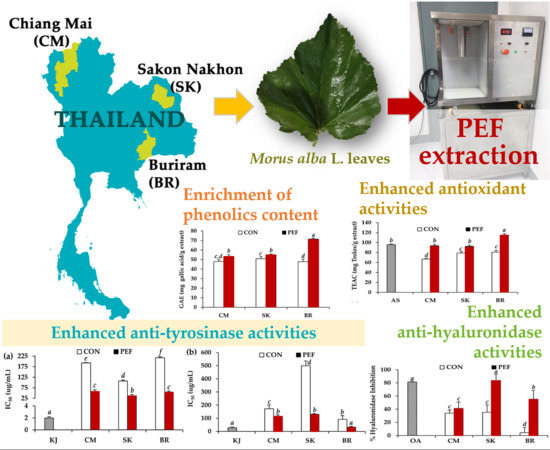
Graphical Abstract
1. Introduction
2. Results and Discussion
2.1. M. alba Leaf Extracts
2.2. Chemical Composition of M. alba Leaf Extracts
2.3. Antioxidant Activity of M. alba Leaf Extract
2.4. Anti-Tyrosinase Activity of M. alba Leaf Extract
2.5. Anti-Hyaluronidase Activity of M. alba Leaf Extract
3. Materials and Methods
3.1. Plant Materials
3.2. Chemical Materials
3.3. Preparation of M. alba Leaf Extract
3.3.1. Maceration Method
3.3.2. PEF Extraction Method
3.4. Determining Chemical Composition of M. alba Leaf Extract
3.4.1. Determining 1-Deoxynojirimycin Content by High-Performance Liquid Chromatography (HPLC)
3.4.2. Determining Total Phenolic Content
3.5. Determining Antioxidant Activity
3.5.1. 2,2′-Diphenyl-1-picrylhydrazyl-hydrate (DPPH) Assay
3.5.2. 2,2′-Azinobis 3-ethylbenzothiazoline-6-sulphonate (ABTS) Assay
3.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6. Determining Anti-Tyrosinase Activity
3.7. Determining Anti-Hyaluronidase Activity
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Devi, B.; Sharma, N.; Kumar, D.; Jeet, K. Morus alba Linn: A phytopharmacological review. Int. J. Pharm. Pharm. Sci. 2013, 5, 14–18. [Google Scholar]
- Qin, C.; Li, Y.; Niu, W.; Ding, Y.; Zhang, R.; Shang, X. Analysis and characterisation of anthocyanins in mulberry fruit. Czech J. Food Sci. 2010, 28, 117–126. [Google Scholar] [CrossRef]
- Khamenei-Tabrizi, A.S.; Sendi, J.J.; Imaani, S.; Shojaee, M. Can Feeding of Silkworm on Different Mulberry Variety Affect Its Performance? J. Econ. Entomol. 2020, 113, 281–287. [Google Scholar] [CrossRef]
- Chen, G.H.; Tong, J.J.; Wang, F.; Hu, X.Q.; Li, X.W.; Tao, F.; Wei, Z.J. Chronic adjunction of 1-deoxynojirimycin protects from age-related behavioral and biochemical changes in the SAMP8 mice. Age 2015, 37, 102. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.J.; Yang, C.H.; Hu, M.L. 1-Deoxynojirimycin inhibits metastasis of B16F10 melanoma cells by attenuating the activity and expression of matrix metalloproteinases-2 and-9 and altering cell surface glycosylation. J. Agric. Food Chem. 2010, 58, 8988–8993. [Google Scholar] [CrossRef]
- Jeanmaire, C.; Danoux, L.; Pauly, G. Glycation during human dermal intrinsic and actinic ageing: An in vivo and in vitro model study. Br. J. Derm. 2001, 145, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Juang, L.J.; Wang, B.S.; Wang, M.Y.; Tai, H.M.; Hung, W.J.; Chen, Y.J.; Huang, M.H. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food Chem. Toxicol. 2011, 49, 785–790. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Chan, K.W.; Sarfraz, R.A.; Uddin, M. Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): A comparative study. Int. J. Mol. Sci. 2012, 13, 6651–6664. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.S.; Lee, H.A.; Lim, Y.; Kim, Y. Mulberry Leaf Extract Inhibits Invasive Potential and Downregulates Hypoxia-Inducible Factor-1α (HIF-1α) in SK-N-BE (2) C Neuroblastoma Cells. Biosci. Biotechnol. Biochem. 2013, 2013, 120763. [Google Scholar] [CrossRef] [Green Version]
- Nowacka, M.; Tappi, S.; Wiktor, A.; Rybak, K.; Miszczykowska, A.; Czyzewski, J.; Drozdzal, K.; Witrowa-Rajchert, D.; Tylewicz, U. The Impact of Pulsed Electric Field on the Extraction of Bioactive Compounds from Beetroot. Foods 2019, 8, 244. [Google Scholar] [CrossRef] [Green Version]
- Mannozzi, C.; Rompoonpol, K.; Fauster, T.; Tylewicz, U.; Romani, S.; Dalla Rosa, M.; Jaeger, H. Influence of Pulsed Electric Field and Ohmic Heating Pretreatments on Enzyme and Antioxidant Activity of Fruit and Vegetable Juices. Foods 2019, 8, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zbinden, M.D.A.; Sturm, B.S.; Nord, R.D.; Carey, W.J.; Moore, D.; Shinogle, H.; Stagg-Williams, S.M. Pulsed electric field (PEF) as an intensification pretreatment for greener solvent lipid extraction from microalgae. Biotechnol. Bioeng. 2013, 110, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Parniakov, O.; Lebovka, N.I.; Van Hecke, E.; Vorobiev, E. Pulsed electric field assisted pressure extraction and solvent extraction from mushroom (Agaricus bisporus). Food Bioprocess Tech. 2014, 7, 174–183. [Google Scholar] [CrossRef]
- Bozinou, E.; Karageorgou, I.; Batra, G.; Dourtoglou, V.G.; Lalas, S.I. Pulsed electric field extraction and antioxidant activity determination of Moringa oleifera dry leaves: A comparative study with other extraction techniques. Beverages 2019, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Ricci, A.; Parpinello, G.P.; Versari, A. Recent advances and applications of pulsed electric fields (PEF) to improve polyphenol extraction and color release during red winemaking. Beverages 2018, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs. 2016, 14, 214. [Google Scholar] [CrossRef]
- Grimi, N.; Dubois, A.; Marchal, L.; Jubeau, S.; Lebovka, N.I.; Vorobiev, E. Selective extraction from microalgae Nannochloropsis sp. using different methods of cell disruption. Bioresour. Technol. 2014, 153, 254–259. [Google Scholar] [CrossRef]
- Luengo, E.; Martínez, J.M.; Bordetas, A.; Álvarez, I.; Raso, J. Influence of the treatment medium temperature on lutein extraction assisted by pulsed electric fields from Chlorella vulgaris. Innov. Food Sci. Emerg. Technol. 2015, 29, 15–22. [Google Scholar] [CrossRef]
- Luengo, E.; Condón-Abanto, S.; Álvarez, I.; Raso, J. Effect of pulsed electric field treatments on permeabilization and extraction of pigments from Chlorella vulgaris. J. Membr. Biol. 2014, 247, 1269–1277. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field and pH assisted selective extraction of intracellular components from microalgae Nannochloropsis. Algal. Res. 2015, 8, 128–134. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs. 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poojary, M.M.; Lund, M.N.; Barba, F.J. Pulsed electric field (PEF) as an efficient technology for food additives and nutraceuticals development. In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Francisco, J.B., Oleksii, P., Artur, W., Eds.; Elsevier Inc.: London, UK, 2020; pp. 65–100. [Google Scholar] [CrossRef]
- Barba, F.J.; Roselló-Soto, E.; Marszałek, K.; Kovačević, D.B.; Jambrak, A.R.; Lorenzo, J.M.; Chemat, F.; Putnik, P. Green food processing: Concepts, strategies, and tools. In Green Food Processing Techniques; Chemat, F., Vorobiev, E., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–21. [Google Scholar] [CrossRef]
- Prabhu, M.S.; Levkov, K.; Livney, Y.D.; Israel, A.; Golberg, A. High-Voltage Pulsed Electric Field Preprocessing Enhances Extraction of Starch, Proteins, and Ash from Marine Macroalgae Ulva ohnoi. Acs Sustain. Chem. Eng. 2019, 7, 17453–17463. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E.; Le, L.H.; Cordin-Falcimaigne, A.; Lanoisellé, J.L. Application of electrical treatments in alcoholic solvent for polyphenols extraction from grape seeds. LWT-Food Sci. Technol. 2012, 46, 127–134. [Google Scholar] [CrossRef]
- Yatsunami, K.; Ichida, M.; Onodera, S. The relationship between 1-deoxynojirimycin content and α-glucosidase inhibitory activity in leaves of 276 mulberry cultivars (Morus spp.) in Kyoto, Japan. J. Nat. Med. 2008, 62, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Jiang, L.; Zhang, J.G.; Deng, W.; Wang, H.L.; Wei, Z.J. Quantitative determination of 1-deoxynojirimycin in mulberry leaves from 132 varieties. Ind. Crop. Prod. 2013, 49, 782–784. [Google Scholar] [CrossRef]
- Boonprong, M. Public policy with promotion to Thai swamp buffaloes production: A case study on raising Thai swamp buffaloes and satisfaction of local farmers in Buriram province, Thailand. In AFBE 2014 conference paper, Proceedings of AFBE 2014 conference paper, Thaksin University, Thailand, 5–6 November 2014; Thaksin University: Songkhla, Thailand, 2014. [Google Scholar]
- Sriprom, M.; Chalvet-Monfray, K.; Chaimane, T.; Vongsawat, K.; Bicout, D.J. Monthly district level risk of dengue occurrences in Sakon Nakhon Province, Thailand. Sci. Total Env. 2010, 408, 5521–5528. [Google Scholar] [CrossRef]
- Doi, R.; Itoh, M.; Chakhatrakan, S.; Uga, S. Epidemiological investigation of parasitic infection of schoolchildren from six elementary schools in Sakon Nakhon Province, Thailand. Kobe J. Med. Sci. 2016, 62, E120. [Google Scholar]
- Tangtrongsup, S.; Scorza, A.V.; Reif, J.S.; Ballweber, L.R.; Lappin, M.R.; Salman, M.D. Seasonal distributions and other risk factors for Giardia duodenalis and Cryptosporidium spp. infections in dogs and cats in Chiang Mai, Thailand. Prev. Vet. Med. 2020, 174, 104820. [Google Scholar] [CrossRef]
- Agcam, E.; Akyıldız, A.; Evrendilek, G.A. Comparison of phenolic compounds of orange juice processed by pulsed electric fields (PEF) and conventional thermal pasteurisation. Food Chem. 2014, 143, 354–361. [Google Scholar] [CrossRef]
- López-Giral, N.; González-Arenzana, L.; González-Ferrero, C.; López, R.; Santamaría, P.; López-Alfaro, I.; Garde-Cerdán, T. Pulsed electric field treatment to improve the phenolic compound extraction from Graciano, Tempranillo and Grenache grape varieties during two vintages. Innov. Food Sci. Emerg. Technol. 2015, 28, 31–39. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K. Application of the pulsed electric field to release bound phenolics in sorghum flour and apple pomace. Innov. Food Sci. Emerg. Technol. 2016, 35, 29–35. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zeng, X.A.; Ngadi, M. Enhanced extraction of phenolic compounds from onion by pulsed electric field (PEF). J. Food Process. Pres. 2018, 42, 13755. [Google Scholar] [CrossRef]
- Fincan, M. Extractability of phenolics from spearmint treated with pulsed electric field. J. Food Eng. 2015, 162, 31–37. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. Current and future prospects for the use of pulsed electric field in the meat industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 1660–1674. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, A.; Singh, A.P.; Parniakov, O.; Mykhailyk, V.; Mandal, R.; Witrowa-Rajchert, D. PEF as an alternative tool to prevent thermolabile compound degradation during dehydration processes. In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Francisco, J.B., Oleksii, P., Artur, W., Eds.; Elsevier Inc.: London, UK, 2020; pp. 155–202. [Google Scholar] [CrossRef]
- Brochier, B.; Mercali, G.D.; Marczak, L.D.F. Effect of moderate electric field on peroxidase activity, phenolic compounds and color during ohmic heating of sugarcane juice. J. Food Process. Preserv. 2019, 43, 14254. [Google Scholar] [CrossRef]
- Li, Y.G.; Ji, D.F.; Zhong, S.; Lv, Z.Q.; Lin, T.B.; Chen, S.; Hu, G.Y. Hybrid of 1-deoxynojirimycin and polysaccharide from mulberry leaves treat diabetes mellitus by activating PDX-1/insulin-1 signaling pathway and regulating the expression of glucokinase, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase in alloxan-induced diabetic mice. J. Ethnopharmacol. 2011, 134, 961–970. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Stuper-Szablewska, K. Phenolic acids used in the cosmetics industry as natural antioxidants. EJMT 2019, 4, 24–32. Available online: http://www.medical-technologies.eu/upload/phenolic_acids_-_przybylska.pdf (accessed on 12 April 2020).
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods. 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Derm. Res. Pr. 2012, 2012, 135206. [Google Scholar] [CrossRef] [Green Version]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winterbourn, C.C. Biological chemistry of superoxide radicals. Chemtexts 2020, 6, 7. [Google Scholar] [CrossRef]
- Igielska-Kalwat, J.; Gościańska, J.; Nowak, I. Carotenoids as natural antioxidants. Post. Hig. Med. Dosw. 2015, 69, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Ferreira, M.; Oliveira, A.S.; Magalhaes, C.; Sousa, M.E.; Pinto, M.; Sousa Lobo, J.M.; Almeida, I.F. Evolution of the use of antioxidants in anti-ageing cosmetics. Int. J. Cos. Sci. 2019, 41, 378–386. [Google Scholar] [CrossRef]
- Hanh, N.T.M.; Phung, N.K.P.; Phuong, Q.N.D. Studying on Tyrosinase Inhibition Activity of Some Vietnamese Folk Plants Aims to Use in Skin-Whitening Cosmetics. Am. J. Plant Sci. 2017, 8, 1319. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Buhren, B.A.; Schrumpf, H.; Hoff, N.P.; Bölke, E.; Hilton, S.; Gerber, P.A. Hyaluronidase: From clinical applications to molecular and cellular mechanisms. Eur. J. Med. Res. 2016, 21, 5. [Google Scholar] [CrossRef] [Green Version]
- Chaiyana, W.; Punyoyai, C.; Somwongin, S.; Leelapornpisid, P.; Ingkaninan, K.; Waranuch, N.; Srivilai, J.; Thitipramote, N.; Wisuitiprot, W.; Schuster, R.; et al. Inhibition of 5α-reductase, IL-6 secretion, and oxidation process of Equisetum debile Roxb. ex vaucher extract as functional food and nutraceuticals ingredients. Nutrients 2017, 9, 1105. [Google Scholar] [CrossRef]
- Saeio, K.; Chaiyana, W.; Okonogi, S. Antityrosinase and antioxidant activities of essential oils of edible Thai plants. Drug Discov. 2011, 5, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Laosirisathian, N.; Saenjum, C.; Sirithunyalug, J.; Eitssayeam, S.; Sirithunyalug, B.; Chaiyana, W. The Chemical Composition, Antioxidant and Anti-Tyrosinase Activities, and Irritation Properties of Sripanya Punica granatum Peel Extract. Cosmetics 2020, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Chaiyana, W.; Anuchapreeda, S.; Punyoyai, C.; Neimkhum, W.; Lee, K.H.; Lin, W.C.; Lue, S.C.; Viernstein, H.; Mueller, M. Ocimum sanctum Linn. as a natural source of skin anti-ageing compounds. Ind. Crops Prod. 2019, 127, 217–224. [Google Scholar] [CrossRef]
Sample Availability: Samples of M. alba leaf extracts are available from the authors. |







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiyana, W.; Sirithunyalug, J.; Somwongin, S.; Punyoyai, C.; Laothaweerungsawat, N.; Marsup, P.; Neimkhum, W.; Yawootti, A. Enhancement of the Antioxidant, Anti-Tyrosinase, and Anti-Hyaluronidase Activity of Morus alba L. Leaf Extract by Pulsed Electric Field Extraction. Molecules 2020, 25, 2212. https://doi.org/10.3390/molecules25092212
Chaiyana W, Sirithunyalug J, Somwongin S, Punyoyai C, Laothaweerungsawat N, Marsup P, Neimkhum W, Yawootti A. Enhancement of the Antioxidant, Anti-Tyrosinase, and Anti-Hyaluronidase Activity of Morus alba L. Leaf Extract by Pulsed Electric Field Extraction. Molecules. 2020; 25(9):2212. https://doi.org/10.3390/molecules25092212
Chicago/Turabian StyleChaiyana, Wantida, Jakkapan Sirithunyalug, Suvimol Somwongin, Chanun Punyoyai, Natnaree Laothaweerungsawat, Pachabadee Marsup, Waranya Neimkhum, and Artit Yawootti. 2020. "Enhancement of the Antioxidant, Anti-Tyrosinase, and Anti-Hyaluronidase Activity of Morus alba L. Leaf Extract by Pulsed Electric Field Extraction" Molecules 25, no. 9: 2212. https://doi.org/10.3390/molecules25092212





