Chemical Composition, Antioxidant and Cytoprotective Potentials of Carica papaya Leaf Extracts: A Comparison of Supercritical Fluid and Conventional Extraction Methods
Abstract
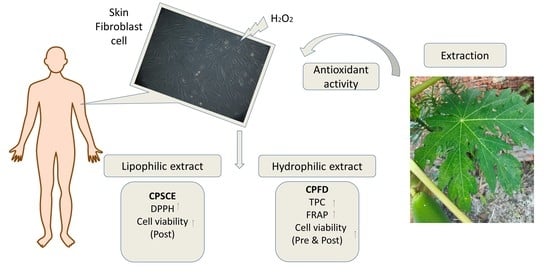
:1. Introduction
2. Results
2.1. GC-MS Analysis of Lipophilic Constituents in CP Leaf Extracts
2.2. HPLC Analysis of Hydrophilic Constituents in CP Leaf Extracts
2.3. Antioxidant Activity of CP Leaf Extract
- (a)
- 1,1-diphenyl-2-picryl-hydrazyl (DPPH) scavenging activity
- (b)
- Total phenolic content (TPC)
- (c)
- Ferric-reduced antioxidative power (FRAP)
2.4. Cytotoxicity of CP Leaf Extracts to Hs27 Human Skin Fibroblasts
2.5. Protective Effect of CP Leaf Extracts towards H2O2-Induced Oxidative Damage
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagent
4.2. Plant Materials
4.3. Extraction Methods
- (a)
- Supercritical fluid extraction
- (b)
- Maceration
- (c)
- Leaf juice extraction
4.4. Chemical Analysis
- (a)
- GC-MS analysis
- (b)
- HPLC profiling of the polar extracts
4.5. Antioxidant Assay
- (a)
- DPPH scavenging activity
- (b)
- Total phenolic content
- (c)
- Ferric-reducing antioxidant power (FRAP)
4.6. Cell Culture and Maintenance
4.7. Cytotoxicity of C. papaya Extracts in Hs27 Human Skin Fibroblasts
4.8. Protective Effect of C. papaya Leaf Extracts Against H2O2 Induced Toxicity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beacham, D.A.; Amatangelo, M.D.; Cukierman, E. Preparation of Extracellular Matrices Produced by Cultured and Primary Fibroblasts. Curr. Protoc. Cell Biol. 2006, 33, 10.9.1–10.9.21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickers, D.R.; Athar, M. Oxidative Stress in the Pathogenesis of Skin Disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gough, D.R.; Cotter, T.G. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death Dis. 2011, 2, e213. [Google Scholar] [CrossRef] [Green Version]
- Milkovic, L.; Gasparovic, A.C.; Cindric, M.; Mouthuy, P.-A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, V.; Calafato, S.; Puleo, E.; Cornelius, C.; Sapienza, M.; Morganti, P.; Mancuso, C. Redox regulation of cellular stress response by ferulic acid ethyl ester in human dermal fibroblasts: Role of vitagenes. Clin. Dermatol. 2008, 26, 358–363. [Google Scholar] [CrossRef]
- Ozbek, E. Induction of Oxidative Stress in Kidney. Int. J. Nephrol. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidoo, K.; Birch-Machin, M.A. Oxidative Stress and Ageing: The Influence of Environmental Pollution, Sunlight and Diet on Skin. Cosmetics 2017, 4, 4. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Canini, A.; Alesiani, D.; D’Arcangelo, G.; Tagliatesta, P. Gas chromatography–mass spectrometry analysis of phenolic compounds from Carica papaya L. leaf. J. Food Compos. Anal. 2007, 20, 584–590. [Google Scholar] [CrossRef]
- Starley, I.F.; Mohammed, P.; Schneider, G.; Bickler, S.W. The treatment of paediatric burns using topical papaya. Burns 1999, 25, 636–639. [Google Scholar] [CrossRef]
- Zunjar, V.; Mammen, D.; Trivedi, B.M.; Daniel, M. Pharmacognostic, Physicochemical and Phytochemical Studies on Carica papaya Linn. Leaves. Pharmacogn. J. 2011, 3, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Nugroho, A.; Heryani, H.; Choi, J.S.; Park, H.-J. Identification and quantification of flavonoids in Carica papaya leaf and peroxynitrite-scavenging activity. Asian Pac. J. Trop. Biomed. 2017, 7, 208–213. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Shaw, P.N.; Parat, M.-O.; Pandey, S.; Falconer, J.R. Compound Identification and In Vitro Cytotoxicity of the Supercritical Carbon Dioxide Extract of Papaya Freeze-Dried Leaf Juice. Processes 2020, 8, 610. [Google Scholar] [CrossRef]
- Soib, H.H.; Ismail, H.F.; Husin, F.; Abu Bakar, M.H.; Yaakob, H.; Sarmidi, M.R. Bioassay-Guided Different Extraction Techniques of Carica papaya (Linn.) Leaves on In Vitro Wound-Healing Activities. Molecules 2020, 25, 517. [Google Scholar] [CrossRef] [Green Version]
- Nayak, B.S.; Ramdeen, R.; Adogwa, A.; Marshall, J.R.; Ramsubhag, A. Wound-healing potential of an ethanol extract of Carica papaya (Caricaceae) seeds. Int. Wound J. 2012, 9, 650–655. [Google Scholar] [CrossRef]
- Gurung, S.; Škalko-Basnet, N. Wound healing properties of Carica papaya latex: In vivo evaluation in mice burn model. J. Ethnopharmacol. 2009, 121, 338–341. [Google Scholar] [CrossRef]
- Anuar, N.S.; Zahari, S.S.; Taib, I.A.; Rahman, M.T. Effect of green and ripe Carica papaya epicarp extracts on wound healing and during pregnancy. Food Chem. Toxicol. 2008, 46, 2384–2389. [Google Scholar] [CrossRef]
- Fitzmaurice, S.; Sivamani, R.; Isseroff, R. Antioxidant Therapies for Wound Healing: A Clinical Guide to Currently Commercially Available Products. Skin Pharmacol. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Roy, S. Redox signals in wound healing. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 1348–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norahmad, N.A.; Razak, M.R.M.A.; Misnan, N.M.; Jelas, N.H.M.; Sastu, U.R.; Muhammad, A.; Ho, T.C.D.; Jusoh, B.; Zolkifli, N.A.; Thayan, R.; et al. Effect of freeze-dried Carica papaya leaf juice on inflammatory cytokines production during dengue virus infection in AG129 mice. BMC Complement. Altern. Med. 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Aguilar-Domínguez, D.E.; La Fuente, L.F.R.-D.; Lobato-García, C.E.; Ble-Castillo, J.L.; López-Meraz, L.; Diaz-Zagoya, J.C.; Bermúdez-Ocaña, D.Y. Phytochemical screening and hypoglycemic activity of Carica papaya leaf in streptozotocin-induced diabetic rats. Rev. Bras. Farmacogn. 2014, 24, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Ilham, R.; Lelo, A.; Harahap, U.; Widyawati, T.; Siahaan, L. The Effectivity of Ethanolic Extract from Papaya Leaves (Carica papaya L.) as an Alternative Larvacide to Aedes spp. Open Access Maced. J. Med. Sci. 2019, 7, 3395–3399. [Google Scholar] [CrossRef] [PubMed]
- Tay, Z.H.; Chong, K.P. The potential of papaya leaf extract in controlling Ganoderma boninense. In Proceedings of the IOP Conference Series: Earth and Environmental Science, International Conference on Chemical and Bioprocess Engineering, Kota Kinabalu, Malaysia, 9–12 December 2015; IOP Publishing Ltd.: Bristol, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Nguyen, T.T.T.; Pandey, S.; Thurecht, K.J.; Falconer, J.R. Factorial design-assisted supercritical carbon-dioxide extraction of cytotoxic active principles from Carica papaya leaf juice. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Chear, N.J.Y.; Maran, S.; Yeong, K.Y.; Ong, Y.S.; Goh, B.H. Butyrylcholinesterase inhibitory activity and GC-MS analysis of Carica papaya leaves. Nat. Prod. Sci. 2020, 26, 165–170. [Google Scholar]
- Subenthiran, S.; Choon, T.C.; Cheong, K.C.; Thayan, R.; Teck, M.B.; Muniandy, P.K.; Afzan, A.; Abdullah, N.R.; Ismail, Z. Carica papaya Leaves Juice Significantly Accelerates the Rate of Increase in Platelet Count among Patients with Dengue Fever and Dengue Haemorrhagic Fever. Evid. Based Complement. Altern. Med. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- A Seo, S.; Ngo, H.T.; Hwang, E.; Park, B.; Yi, T.-H. Protective effects of Carica papaya leaf against skin photodamage by blocking production of matrix metalloproteinases and collagen degradation in UVB-irradiated normal human dermal fibroblasts. S. Afr. J. Bot. 2020, 131, 398–405. [Google Scholar] [CrossRef]
- Mahmood, A.A.; Sidik, K.; Salmah, I. Wound Healing Activity of Carica papaya L. Aqueous Leaf Extract in Rats. Int. J. Mol. Med. Adv. Sci. 2005, 1, 398–401. [Google Scholar]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Bilgic, M.B.; Lacin, N.T.; Berber, H.; Mansuroglu, B. In vitro evaluation of alpha-tocopherol loaded carboxymethylcellulose chitosan copolymers as wound dressing materials. Mater. Technol. 2019, 34, 1–8. [Google Scholar] [CrossRef]
- Lopez-Torres, M.; Thiele, J.J.; Shindo, Y.; Han, D.; Packer, L. Topical application of α-tocopherol modulates the antioxidant network and diminishes ultraviolet-induced oxidative damage in murine skin. Br. J. Dermatol. 1998, 138, 207–215. [Google Scholar] [CrossRef]
- Gordon, M.H.; Magos, P. The effect of sterols on the oxidation of edible oils. Food Chem. 1983, 10, 141–147. [Google Scholar] [CrossRef]
- Lucero, M.; Vigo, J.; Leon, M.; Martin, F.; Sánchez, J. Therapeutic efficacy of hydrophilic gels of α-tocopherol and tretinoin in skin ulcers induced by adriamycin hydrochloride. Int. J. Pharm. 1996, 127, 73–83. [Google Scholar] [CrossRef]
- Zahid, S.; Khalid, H.; Ikram, F.; Iqbal, H.; Samie, M.; Shahzadi, L.; Shah, A.T.; Yar, M.; Chaudhry, A.A.; Awan, S.J.; et al. Bi-layered α-tocopherol acetate loaded membranes for potential wound healing and skin regeneration. Mater. Sci. Eng. C 2019, 101, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Micera, M.; Botto, A.; Geddo, F.; Antoniotti, S.; Bertea, C.M.; Levi, R.; Gallo, M.P.; Querio, G. Squalene: More than a Step toward Sterols. Antioxidants 2020, 9, 688. [Google Scholar] [CrossRef]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Liang, T.; Yue, W.; Li, Q. Comparison of the Phenolic Content and Antioxidant Activities of Apocynum venetum L. (Luo-Bu-Ma) and Two of Its Alternative Species. Int. J. Mol. Sci. 2010, 11, 4452–4464. [Google Scholar] [CrossRef]
- Kono, Y.; Kobayashi, K.; Tagawa, S.; Adachi, K.; Ueda, A.; Sawa, Y.; Shibata, H. Antioxidant activity of polyphenolics in diets. Biochim. Biophys. Acta Gen. Subj. 1997, 1335, 335–342. [Google Scholar] [CrossRef]
- Malaysian Herbal Monograph: Carica papaya L. Available online: https://www.globinmed.com/index.php?option=com_content&view=article&id=105958:carica-papaya-l-105958&catid=209&Itemid=143 (accessed on 30 January 2021).
- Tran, N.Q.; Joung, Y.K.; Lih, E.; Park, K.D. In Situ Forming and Rutin-Releasing Chitosan Hydrogels as Injectable Dressings for Dermal Wound Healing. Biomacromolecules 2011, 12, 2872–2880. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Huang, C.-N.; Liao, C.-K.; Chang, H.-M.; Kuan, Y.-H.; Tseng, T.-J.; Yen, K.-J.; Yang, K.-L.; Lin, H.-C. Effects of Rutin on Wound Healing in Hyperglycemic Rats. Antioxidants 2020, 9, 1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, Y.; Yang, W.; Yang, Z.; Jiang, S.; Zhang, C.; Zhang, G. Alfalfa polysaccharide prevents H2O2-induced oxidative damage in MEFs by activating MAPK/Nrf2 signaling pathways and suppressing NF-κB signaling pathways. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shen, T.; Duan, C.; Chen, B.; Li, M.; Ruan, Y.; Xu, D.; Shi, D.; Yu, D.; Li, J.; Wang, C. Tremella fuciformis polysaccharide suppresses hydrogen peroxide-triggered injury of human skin fibroblasts via upregulation of SIRT1. Mol. Med. Rep. 2017, 16, 1340–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chear, N.J.-Y.; Khaw, K.-Y.; Murugaiyah, V.; Lai, C.-S. Cholinesterase inhibitory activity and chemical constituents of Stenochlaena palustris fronds at two different stages of maturity. J. Food Drug Anal. 2016, 24, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Chear, N.J.-Y.; Fauzi, A.N.; Khaw, K.-Y.; Choi, S.-B.; Yaacob, N.S.; Lai, C.-S. Free Radical Scavenging and Cytotoxic Properties of Acylated and Non-Acylated Kaempferol Glycosides from Stenochlaena Palustris: A Perspective on Their Structure – Activity Relationships. Pharm. Chem. J. 2019, 53, 188–193. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Santos, J.S.; Brizola, V.R.A.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Ponnusamy, Y.; Chear, N.J.-Y.; Ramanathan, S.; Lai, C.-S. Polyphenols rich fraction of Dicranopteris linearis promotes fibroblast cell migration and proliferation in vitro. J. Ethnopharmacol. 2015, 168, 305–314. [Google Scholar] [CrossRef] [PubMed]









| Peak Label | Identified Compound | Retention Time (min) | * Corresponding % Maximum Based on Peak Area | ||||
|---|---|---|---|---|---|---|---|
| CPHE | CPFD | CPEE | CPSC | CPSCE | |||
| 1 | Hexadecanoic acid | 10.68 | 100 | 12.97 | 11.52 | 39.60 | 33.97 |
| 2 | Phytol | 11.61 | ND | ND | ND | 78.49 | 100 |
| 3 | Squalene | 15.55 | 27.15 | 4.88 | 13.72 | 54.19 | 100 |
| 4 | γ-tocopherol | 18.07 | ND | ND | ND | 100 | 97.76 |
| 5 | α-tocopherol | 19.29 | 26.42 | 2.11 | 14.61 | 19.43 | 100 |
| 6 | Campesterol | 21.07 | 37.56 | ND | ND | 100 | 78.89 |
| 7 | Stigmasterol | 21.67 | ND | ND | ND | 85.10 | 100 |
| 8 | β-sitosterol | 22.93 | 54.39 | ND | 26.33 | 100 | 93.53 |
| 9 | Olean-12-ene | 23.19 | ND | ND | ND | 100 | ND |
| 10 | 13,17-cycloursan-3-one | 24.30 | ND | ND | ND | 33.89 | 100 |
| 11 | Cycloartenol | 24.66 | 86.35 | ND | ND | 78.97 | 100 |
| 12 | Carpaine | 29.65 | ND | ND | 100 | ND | ND |
| CP Leaf Extract | IC50 (μg/mL) |
|---|---|
| CPHE | 459.86 ± 11.91 |
| CPFD | 398.03 ± 31.84 |
| CPEE | 151.36 ± 4.38 |
| CPSC | 92.32 ± 3.58 |
| CPSCE | 69.05 ± 11.47 |
| Butylated hydroxytoluene (standard) | 89.1 ± 0.90 |
| Time | % A (0.1% Formic Acid) | % B (Acetonitrile) |
|---|---|---|
| 5.0 | 90 | 10 |
| 20.0 | 70 | 30 |
| 22.0 | 5 | 95 |
| 25.0 | 5 | 95 |
| 26.0 | 90 | 10 |
| 30 | 90 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khor, B.-K.; Chear, N.J.-Y.; Azizi, J.; Khaw, K.-Y. Chemical Composition, Antioxidant and Cytoprotective Potentials of Carica papaya Leaf Extracts: A Comparison of Supercritical Fluid and Conventional Extraction Methods. Molecules 2021, 26, 1489. https://doi.org/10.3390/molecules26051489
Khor B-K, Chear NJ-Y, Azizi J, Khaw K-Y. Chemical Composition, Antioxidant and Cytoprotective Potentials of Carica papaya Leaf Extracts: A Comparison of Supercritical Fluid and Conventional Extraction Methods. Molecules. 2021; 26(5):1489. https://doi.org/10.3390/molecules26051489
Chicago/Turabian StyleKhor, Boon-Keat, Nelson Jeng-Yeou Chear, Juzaili Azizi, and Kooi-Yeong Khaw. 2021. "Chemical Composition, Antioxidant and Cytoprotective Potentials of Carica papaya Leaf Extracts: A Comparison of Supercritical Fluid and Conventional Extraction Methods" Molecules 26, no. 5: 1489. https://doi.org/10.3390/molecules26051489