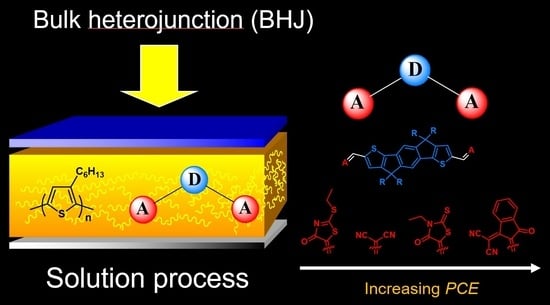
Effect of the Terminal Acceptor Unit on the Performance of Non-Fullerene Indacenodithiophene Acceptors in Organic Solar Cells
Abstract
:1. Introduction
2. Results
2.1. Synthesis
2.2. Theoretical Calculations
2.3. Electrochemical and Optical Properties
2.4. Photovoltaic Properties
2.5. Dipole Moment Calculations
3. Experimental Section
3.1. General
3.2. Synthesis of Compounds
3.3. Device Fabrication and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, P.; Yang, Y. Narrowing the Band Gap: The Key to High-Performance Organic Photovoltaics. Acc. Chem. Res. 2020, 53, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wang, J.; Xu, Y.; Zhang, S.; Hou, J. Recent Progress in Chlorinated Organic Photovoltaic Materials. Acc. Chem. Res. 2020, 53, 822–832. [Google Scholar] [CrossRef]
- Inganäs, O. Organic Photovoltaics over Three Decades. Adv. Mater. 2018, 30, 1800388–1800413. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Yu, K.; Someya, T. The Future of Flexible Organic Solar Cells. Adv. Energy Mater. 2020, 10, 2000765–2000774. [Google Scholar] [CrossRef]
- Qin, J.; Lan, L.; Chen, S.; Huang, F.; Shi, H.; Chen, W.; Xia, H.; Sun, K.; Yang, C. Recent Progress in Flexible and Stretchable Organic Solar Cells. Adv. Funct. Mater. 2020, 30, 2002529–2002550. [Google Scholar] [CrossRef]
- Wadsworth, A.; Hamid, Z.; Kosco, J.; Gasparini, N.; McCulloch, I. The Bulk Heterojunction in Organic Photovoltaic, Photodetector, and Photocatalytic Applications. Adv. Mater. 2020, 32, 2001763–2001789. [Google Scholar] [CrossRef]
- Rafique, S.; Abdullah, S.M.; Sulaiman, K.; Iwamoto, M. Fundamentals of bulk heterojunction organic solar cells: An overview of stability/degradation issues and strategies for improvement. Renew. Sustain. Energy Rev. 2018, 84, 43–53. [Google Scholar] [CrossRef]
- Usmani, B.; Ranjan, R.; Gupta, S.K.; Gupta, R.K.; Nalwa, K.S.; Garg, A. Inverted PTB7-Th:PC71BM organic solar cells with 11.8% PCE via incorporation of gold nanoparticles in ZnO electron transport layer. Sol. Energy 2021, 214, 220–230. [Google Scholar] [CrossRef]
- Liang, X.; Wang, J.; Miao, R.; Zhao, Q.; Huang, L.; Wen, S.; Tang, J. The evolution of small molecular acceptors for organic solar cells: Advances, challenges and prospects. Dye. Pigm. 2022, 198, 109963. [Google Scholar] [CrossRef]
- Li, C.; Zhou, J.; Song, J.; Xu, J.; Zhang, H.; Zhang, X.; Guo, J.; Zhu, L.; Wei, D.; Han, G.; et al. Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells. Nat. Energy 2021, 6, 605–613. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular optimization enables over 13% efficiency in organic solar cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Singh, S.P. An indacenodithiophene core moiety for organic solar cells. Mater. Chem. Front. 2021, 5, 7724–7736. [Google Scholar] [CrossRef]
- Khlaifia, D.; Ettaghzouti, T.; Chemek, M.; Alimi, K. Indacenodithiophene (IDT) and indacenodithienothiophene (IDTT)-based acceptors for non-fullerene organic solar cells. Synth. Met. 2021, 274, 116736–116757. [Google Scholar] [CrossRef]
- Forti, G.; Nitti, A.; Osw, P.; Bianchi, G.; Po, R.; Pasini, D. Recent Advances in Non-Fullerene Acceptors of the IDIC/ITIC Families for Bulk-Heterojunction Organic Solar Cells. Int. J. Mol. Sci. 2020, 21, 8085. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, Y.J.; Lee, Y.W.; Koh, C.W.; Lee, Y.; Kim, M.J.; Liao, K.; Cho, J.H.; Kim, B.J.; Woo, H.Y. Impact of Terminal End-Group of Acceptor-Donor-Acceptor-type Small Molecules on Molecular Packing and Photovoltaic Properties. ACS Appl. Mater. Interfaces 2017, 10, 39952–39961. [Google Scholar] [CrossRef]
- Diac, A.; Demeter, D.; Allain, M.; Grosu, I.; Roncali, J. Simple and Versatile Molecular Donors for Organic Photovoltaics Prepared by Metal-Free Synthesis. Chem. Eur. J. 2015, 21, 1598–1608. [Google Scholar] [CrossRef]
- Xia, T.; Li, C.; Ryu, H.S.; Guo, J.; Min, J.; Woo, H.Y.; Sun, Y. Efficient Fused-Ring Extension of A–D–A-Type Non-Fullerene Acceptors by a Symmetric Replicating Core Unit Strategy. Chem. Eur. J. 2020, 26, 12411–12417. [Google Scholar] [CrossRef]
- Ryu, H.S.; Lee, H.G.; Shin, S.-C.; Park, J.; Kim, S.H.; Kim, E.J.; Shin, T.J.; Shim, J.W.; Kim, B.J.; Woo, H.J. Terminal alkyl substitution in an A–D–A-type nonfullerene acceptor: Simultaneous improvements in the open-circuit voltage and short-circuit current for efficient indoor power generation. J. Mater. Chem. A 2020, 8, 23894–23905. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.; Cheng, P.; Wang, J.; Wu, Y.; Hou, J.; Zhan, X. An electron acceptor based on indacenodithiophene and 1,1-dicyanomethylene-3-indanone for fullerene-free organic solar cells. J. Mater. Chem. A 2015, 3, 1910–1914. [Google Scholar] [CrossRef]
- Ming, S.; Liu, Y.; Feng, S.; Jiang, P.; Zhang, C.; Li, M.; Song, J.; Bo, Z. Fused-ring acceptor with a spiro-bridged ladder-type core for organic solar cells. Dye. Pigment. 2019, 163, 153–158. [Google Scholar] [CrossRef]
- Che, Y.; Zhang, Y.; Yang, Y.; Liu, C.-H.; Izquierdo, R.; Shuyong Xiao, S.; Perepichka, D.F. Understanding the Photovoltaic Behavior of A−D−A Molecular Semiconductors through a Permutation of End Groups. J. Org. Chem. 2020, 85, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Hou, L.; Fu, J.; Kan, Z.; Yang, Q.; Chen, Q.; Zhong, C.; Xiao, Z.; Yu, D.; Lu, S. An asymmetric end-capping strategy enables a new non-fullerene acceptor for organic solar cells with efficiency over 10. Chem. Commun. 2020, 56, 6531–6534. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.M.; Xiao, J.Y.; Bai, W.Y.; Li, Q.Y.; Wang, H.C.; Miao, M.S.; Yip, H.L.; Xu, Y.X. End-chain effects of non-fullerene acceptors on polymer solar cells. Org. Electron. 2019, 64, 1–6. [Google Scholar] [CrossRef]
- Terenti, N.; Crisan, A.P.; Jungsuttiwong, S.; Hadade, N.D.; Pop, A.; Grosu, I.; Roncali, J. Effect of the mode of fixation of the thienyl rings on the electronic properties of electron acceptors based on indacenodithiophene (IDT). Dye. Pigm. 2021, 187, 109116–109124. [Google Scholar] [CrossRef]
- Lin, Y.; Li, T.; Zhao, F.; Han, L.; Wang, Z.; Wu, Y.; He, Q.; Wang, J.; Huo, L.; Sun, Y.; et al. Structure Evolution of Oligomer Fused-Ring Electron Acceptors toward High Efficiency of As-Cast Polymer Solar Cells. Adv. Energy Mater. 2016, 6, 1600854–1600862. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, S.; He, C.; Zhang, J.; Yang, Y.; Zhu, J.; Cui, Y.; Zhao, W.; Zhang, H.; Zhang, Y.; et al. Modulating Molecular Orientation Enables Efficient Non-Fullerene Small-Molecule Organic Solar Cells. Chem. Mater. 2018, 30, 2129–2134. [Google Scholar] [CrossRef]
- Bello, K.A.; Cheng, L.; Griffiths, J. Near-infrared Absorbing Methine Dyes based on Dicyanovinyl Derivatives of Indane-1,3-dione. J. Chem. Soc. Perkin Trans. 1987, 2, 815–818. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154122. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent Molecular Orbital Methods 25. Supplementary Functions for Gaussian Basis Sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Ko, E.Y.; Park, G.E.; Lee, J.H.; Kim, H.J.; Lee, D.H.; Ahn, H.; Uddin, M.A.; Woo, H.Y.; Cho, M.J.; Choi, D.H. Excellent Long-Term Stability of Power Conversion Efficiency in Non-Fullerene-Based Polymer Solar Cells Bearing Tricyanovinylene-Functionalized n-Type Small Molecules. ACS Appl. Mater. Interfaces 2017, 9, 8838–8847. [Google Scholar] [CrossRef]
- Xin, H.; Subramaniyan, S.; Kwon, T.-W.; Shoaee, S.; Durrant, J.R.; Jenekhe, S.A. Enhanced Open Circuit Voltage and Efficiency of Donor−Acceptor Copolymer Solar Cells by Using Indene-C60 Bisadduct. Chem. Mater. 2012, 24, 1995–2001. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted polymer solar cells integrated with a low-temperature-annealed sol-gel-derived ZnO film as an electron transport layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Brabec, C.J.; Cravino, A.; Meissner, D.; Sariciftci, N.S.; Fromhertz, T.; Rispens, M.T.; Sanchess, L.; Hummelen, J.C. Origin of the open circuit voltage of plastic solar cells. Adv. Funct. Mater. 2001, 11, 374–380. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wu, F.-P.; Zhou, Y.; Jiang, Z.-Q.; Song, B.; Xia, Y.; Zhang, Z.H.; Gao, F.; Inganas, O.; et al. Non-fullerene acceptor with low energy loss and high external quantum efficiency: Towards high performance polymer solar cells. J. Mater. Chem. A 2016, 4, 5890–5897. [Google Scholar] [CrossRef]
- Kranthiraja, K.; Kim, S.; Lee, C.; Gunasekar, K.; Sree, V.G.; Gautam, B.; Gundogdu, K.; Jin, S.-H.; Kim, B.J. The Impact of Sequential Fluorination of π-Conjugated Polymers on Charge Generation in All-Polymer Solar Cells. Adv. Funct. Mater. 2017, 27, 1701256–1701263. [Google Scholar] [CrossRef]
- Lu, L.; Yu, L. Understanding Low Bandgap Polymer PTB7 and Optimizing Polymer Solar Cells Based on It. Adv. Mater. 2014, 26, 4413–4430. [Google Scholar] [CrossRef]






| IDT-1 | IDT-2 | IDT-3 | IDT-4 | |
|---|---|---|---|---|
| optical | ||||
| λmax a [nm] | 495, 527 | 523, 555 | 505, 533 | 598 (sh), 645 |
| E [ev] b | 2.18 | 2.03 | 2.10 | 1.74 |
| λmax c [nm] | 502, 540 | 530, 576 | 506, 544 | 620, 686 |
| Egopt [ev] d | 2.13 | 2.04 | 2.10 | 1.70 |
| electrochemical | ||||
| Epa [V] | 0.89 | 0.72 | 0.72 | 0.90 |
| Epc1, Epc2 [V] | −1.43, −1.67 | −1.63 | −1.95 | −1.22, −1.40 |
| EHOMO [eV] e | −5.85 | −5.68 | −5.66 | −5.86 |
| ELUMO [eV] e | −3.85 | −3.60 | −3.62 | −4.00 |
| Eg [eV] e | 2.00 | 2.08 | 2.04 | 1.86 |
| B3LYP-D3/6-311G(d,p) | ||||
| EHOMO [eV] f | −6.00 | −5.60 | −5.52 | −5.86 |
| ELUMO [eV] f | −3.47 | −3.22 | −3.04 | −3.65 |
| Eg [eV] | 2.53 | 2.38 | 2.48 | 2.21 |
| n-Type Molecule | P3HT/n-Type Molecule Ratio (wt %) | Active Layer Thickness (nm) | Annealing T (°C) | Voc (V) | Jsc (mAcm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|---|---|
| IDT-1 | 1:2 | 70 | - | 0.67 (0.65) | 1.76 (1.71) | 46.32 (46.71) | 0.55 (0.52) |
| IDT-2 | 1:1 | 80 | - | 0.97 (0.95) | 3.91 (3.79) | 45.56 (45.00) | 1.74 (1.67) |
| IDT-3 | 1:2 | 60 | - | 0.79 (0.75) | 0.89 (0.85) | 41.31 (38.75) | 0.29 (0.26) |
| IDT-4 | 1:1 | 80 | - | 0.54 (0.53) | 7.23 (7.15) | 56.53 (56.04) | 2.21 (2.16) |
| PC60BM | 1:1 | 80 | - | 0.58 (0.57) | 3.32 (2.77) | 17.45 (15.56) | 0.34 (0.26) |
| PC60BM | 1:1 | 80 | 130 | 0.51(0.49) | 6.44 (6.05) | 52.98 (50.30) | 1.72 (1.51) |
| Compound | µg (D) | µe (D) | Δµge (D) |
|---|---|---|---|
| IDT-1 | 0.16 | 0.17 | 0.02 |
| IDT-2 | 0.40 | 0.43 | 0.03 |
| IDT-3 | 0.01 | 0.03 | 0.03 |
| IDT-4 | 0.39 | 0.45 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terenti, N.; Giurgi, G.-I.; Szolga, L.; Stroia, I.; Terec, A.; Grosu, I.; Crișan, A.P. Effect of the Terminal Acceptor Unit on the Performance of Non-Fullerene Indacenodithiophene Acceptors in Organic Solar Cells. Molecules 2022, 27, 1229. https://doi.org/10.3390/molecules27041229
Terenti N, Giurgi G-I, Szolga L, Stroia I, Terec A, Grosu I, Crișan AP. Effect of the Terminal Acceptor Unit on the Performance of Non-Fullerene Indacenodithiophene Acceptors in Organic Solar Cells. Molecules. 2022; 27(4):1229. https://doi.org/10.3390/molecules27041229
Chicago/Turabian StyleTerenti, Natalia, Gavril-Ionel Giurgi, Lorant Szolga, Ioan Stroia, Anamaria Terec, Ion Grosu, and Andreea Petronela Crișan. 2022. "Effect of the Terminal Acceptor Unit on the Performance of Non-Fullerene Indacenodithiophene Acceptors in Organic Solar Cells" Molecules 27, no. 4: 1229. https://doi.org/10.3390/molecules27041229