Bio-Inspired Rhamnolipids, Cyclic Lipopeptides and a Chito-Oligosaccharide Confer Protection against Wheat Powdery Mildew and Inhibit Conidia Germination
Abstract
:1. Introduction
2. Results
2.1. Characterization of the COS BioA187
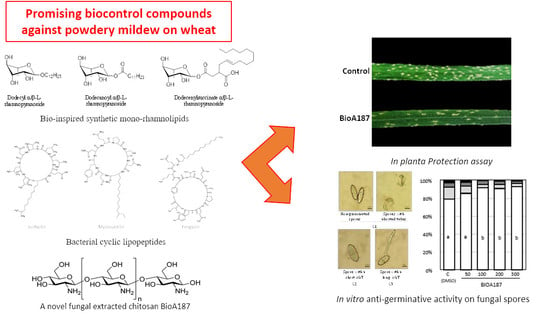
2.2. The COS BioA187, the Three Bio-Inspired smRLs and Mixtures of CLPs Conferred Partial Protections of Wheat against B. graminis f.sp. tritici
2.3. Antigerminative Effect of COS BioA187, the Three Bio-Inspired smRLs and CLPs on B. graminis f.sp. tritici Spores
3. Discussion
4. Materials and Methods
4.1. Bio-Inpired Synthetic Mono-Rhamnolipids (smRLs)

4.2. Production and Characterization of the COS BioA187

4.3. Production and Purification of LPs

4.4. Biological Material
4.5. Plant Treatment, Pathogen Inoculation and Disease Evaluation
4.6. In Vitro Antifungal Assay
4.7. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paux, E.; Sourdille, P.; Salse, J.; Saintenac, C.; Choulet, F.; Leroy, P.; Korol, A.; Michalak, M.; Kianian, S.; Spielmeyer, W.; et al. A physical map of the 1-gigabase bread wheat chromosome 3B. Science 2008, 322, 101–104. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization of the United States). How to Feed the World in 2050. Available online: www.fao.org (accessed on 6 May 2022).
- Langridge, P. Decoding Our daily bread. Nature 2012, 491, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Niu, J.; Li, S. Impacts of wheat powdery mildew on grain yield & quality and its prevention and control methods. Am. J. Agric. For. 2018, 6, 141–147. [Google Scholar]
- Reis, E.M.; Basso, D.F.; Zanatta, M. Loss of Sensitivity of Blumeria graminis f.sp. tritici to triadimenol applied as seed treatment. Trop. Plant Pathol. 2013, 38, 55–57. [Google Scholar]
- Broscious, S.C. Influence of winter wheat management practices on the severity of powdery mildew and Septoria Blotch in Pennsylvania. Phytopathology 1985, 75, 538. [Google Scholar] [CrossRef]
- Burdon, J.J.; Chilvers, G.A. Host density as a factor in plant disease ecology. Annu. Rev. Phytopathol. 1982, 20, 143–166. [Google Scholar] [CrossRef]
- Tang, S.; Hu, Y.; Zhong, S.; Luo, P. The potential role of powdery mildew-resistance gene Pm40 in Chinese wheat-breeding programs in the post-Pm21 Era. Engineering 2018, 4, 500–506. [Google Scholar] [CrossRef]
- Vargas, J.M. A Benzimidazole resistant strain of Erysiphe graminis. Phytopathology 1973, 63, 1366–1368. [Google Scholar] [CrossRef]
- Buchenauer, H.; Hellwald, K.-H. Resistance of Erysiphe graminis on barley and wheat to sterol C-14-demethylation inhibitors1. EPPO Bull. 1985, 15, 459–466. [Google Scholar] [CrossRef]
- Fraaije, B.A.; Butters, J.A.; Coelho, J.M.; Jones, D.R.; Hollomon, D.W. Following the dynamics of strobilurin resistance in Blumeria graminis f.sp. tritici using quantitative allele-specific real-time PCR measurements with the fluorescent dye SYBR Green I. Plant Pathol. 2002, 51, 45–54. [Google Scholar]
- Ritter, L.; Goushleff, N.C.I.; Arbuckle, T.; Cole, D.; Raizenne, M. Addressing the Linkage between Exposure to Pesticides and Human health effects—Research trends and priorities for research. J. Toxicol. Environ. Health Part B 2006, 9, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Itoiz, E.S.; Fantke, P.; Juraske, R.; Kounina, A.; Vallejo, A.A. Deposition and residues of azoxystrobin and imidacloprid on greenhouse lettuce with implications for human consumption. Chemosphere 2012, 89, 1034–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caylak, E.; Tokar, M. Investigating chemical and microbiological contaminants in drinking water of Cankiri province, Turkey. Environ. Earth Sci. 2012, 67, 2015–2025. [Google Scholar] [CrossRef]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boller, T.; Felix, G. A Renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, S.; Héloir, M.-C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [Green Version]
- Crouzet, J.; Arguelles-Arias, A.; Dhondt-Cordelier, S.; Cordelier, S.; Pršić, J.; Hoff, G.; Mazeyrat-Gourbeyre, F.; Baillieul, F.; Clément, C.; Ongena, M.; et al. Biosurfactants in plant protection against diseases: Rhamnolipids and lipopeptides sase study. Front. Bioeng. Biotechnol. 2020, 8, 1014. [Google Scholar] [CrossRef]
- Moussian, B. Chitin: Structure, Chemistry and Biology. In Targeting Chitin-Containing Organisms; Yang, Q., Fukamizo, T., Eds.; Springer: Singapore, 2019; pp. 5–18. [Google Scholar]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; de Lima, M.A.B.; de Oliveira Franco, L.; de Campos-Takaki, G.M. Seafood waste as attractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef] [Green Version]
- Miya, A.; Albert, P.; Shinya, T.; Desaki, Y.; Ichimura, K.; Shirasu, K.; Narusaka, Y.; Kawakami, N.; Kaku, H.; Shibuya, N. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 19613–19618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.; Tanaka, K.; Zhang, X.-C.; Son, G.H.; Brechenmacher, L.; Nguyen, T.H.N.; Stacey, G. LYK4, a Lysin motif receptor-like kinase, is important for chitin signaling and plant innate immunity in Arabidopsis. Plant Physiol. 2012, 160, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liang, Y.; Tanaka, K.; Nguyen, C.T.; Jedrzejczak, R.P.; Joachimiak, A.; Stacey, G. The kinase LYK5 is a major chitin receptor in Arabidopsis and forms a chitin-induced complex with related kinase CERK1. eLife 2014, 3, e03766. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, Z.; Song, C.; Hu, Y.; Han, Z.; She, J.; Fan, F.; Wang, J.; Jin, C.; Chang, J.; et al. Chitin-induced dimerization activates a plant immune receptor. Science 2012, 336, 1160–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaku, H.; Nishizawa, Y.; Ishii-Minami, N.; Akimoto-Tomiyama, C.; Dohmae, N.; Takio, K.; Minami, E.; Shibuya, N. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 11086–11091. [Google Scholar] [CrossRef] [Green Version]
- Ndimba, B.K.; Chivasa, S.; Hamilton, J.M.; Simon, W.J.; Slabas, A.R. Proteomic analysis of changes in the extracellular matrix of Arabidopsis cell suspension cultures induced by fungal elicitors. Proteomics 2003, 3, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, X.; Yang, J.; Yin, H.; Wang, W.; Lu, H.; Du, Y. Nitric oxide production and its functional link with OIPK in tobacco defense response elicited by chitooligosaccharide. Plant Cell Rep. 2011, 30, 1153–1162. [Google Scholar] [CrossRef]
- Klüsener, B.; Young, J.J.; Murata, Y.; Allen, G.J.; Mori, I.C.; Hugouvieux, V.; Schroeder, J.I. Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol. 2002, 130, 2152–2163. [Google Scholar] [CrossRef] [Green Version]
- Rakwal, R.; Tamogami, S.; Agrawal, G.K.; Iwahashi, H. Octadecanoid signaling component “burst” in rice (Oryza sativa L.) seedling leaves upon wounding by cut and treatment with fungal elicitor chitosan. Biochem. Biophys. Res. Commun. 2002, 295, 1041–1045. [Google Scholar] [CrossRef]
- Jia, X.; Meng, Q.; Zeng, H.; Wang, W.; Yin, H. Chitosan oligosaccharide induces resistance to tobacco mosaic virus in Arabidopsis via the salicylic acid-mediated signalling pathway. Sci. Rep. 2016, 6, 26144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcón-Rodríguez, A.B.; Costales, D.; Cabrera, J.C.; Martínez-Téllez, M.Á. Chitosan physico–chemical properties modulate defense responses and resistance in tobacco plants against the oomycete Phytophthora nicotianae. Pestic. Biochem. Physiol. 2011, 100, 221–228. [Google Scholar] [CrossRef]
- Ghaouth, A.E. Antifungal activity of chitosan on two postharvest pathogens of strawberry fruits. Phytopathology 1992, 82, 398. [Google Scholar] [CrossRef]
- Ben-Shalom, N.; Ardi, R.; Pinto, R.; Aki, C.; Fallik, E. Controlling gray mould caused by Botrytis cinerea in cucumber plants by means of chitosan. Crop Prot. 2003, 22, 285–290. [Google Scholar] [CrossRef]
- Ramos Berger, L.R.; Montenegro Stamford, T.C.; de Oliveira, K.Á.R.; de Miranda Pereira Pessoa, A.; de Lima, M.A.B.; Estevez Pintado, M.M.; Saraiva Câmara, M.P.; de Oliveira Franco, L.; Magnani, M.; de Souza, E.L. Chitosan produced from Mucorales fungi using agroindustrial by-products and its efficacy to inhibit colletotrichum species. Int. J. Biol. Macromol. 2018, 108, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zuo, J.; Wang, Q.; Na, Y.; Gao, L. Inhibitory effect of chitosan on growth of the fungal phytopathogen, Sclerotinia sclerotiorum, and Sclerotinia rot of carrot. J. Integr. Agric. 2015, 14, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Palma-Guerrero, J.; Jansson, H.-B.; Salinas, J.; Lopez-Llorca, L.V. Effect of chitosan on hyphal growth and spore germination of plant pathogenic and biocontrol fungi. J. Appl. Microbiol. 2008, 104, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Shovan, L.R.; Hjeljord, L.G.; Aam, B.B.; Eijsink, V.G.H.; Sørlie, M.; Tronsmo, A. Inhibition of fungal plant pathogens by synergistic action of chito-oligosaccharides and commercially available fungicides. PLoS ONE 2014, 9, e93192. [Google Scholar] [CrossRef]
- Hassni, M.; Hadrami, A.E.; Daayf, F.; Barka, E.A.; Hadrami, I.E. Chitosan, antifungal product against Fusarium oxysporum f.sp. albedinis and elicitor of defence reactions in date palm roots. Phytopathol. Mediterr. 2004, 43, 195–204. [Google Scholar]
- Hernández-Lauzardo, A.N.; Velàzquez-del Valle, M.G.; Guerra-Sanchez, M.G. Current status of action mode and effect of chitosan against phytopathogens fungi. Afr. J. Microbiol. Res. 2011, 5, 4243–4247. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- D’aes, J.; De Maeyer, K.; Pauwelyn, E.; Höfte, M. Biosurfactants in plant–Pseudomonas interactions and their importance to biocontrol. Environ. Microbiol. Rep. 2010, 2, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Xun-Chao, C.; Hui, L.; Ya-Rong, X.; Chang-Hong, L. Study of endophytic Bacillus amyloliquefaciens CC09 and its antifungal cyclic lipopeptides. J. Appl. Biol. Biotechnol. 2013, 1, 1–5. [Google Scholar]
- Guo, Q.; Dong, W.; Li, S.; Lu, X.; Wang, P.; Zhang, X.; Wang, Y.; Ma, P. Fengycin produced by Bacillus subtilis NCD-2 plays a major role in biocontrol of cotton seedling damping-off disease. Microbiol. Res. 2014, 169, 533–540. [Google Scholar] [CrossRef]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Jourdan, E.; Henry, G.; Duby, F.; Dommes, J.; Barthélemy, J.P.; Thonart, P.; Ongena, M. Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol. Plant Microbe Interac. 2009, 22, 456–468. [Google Scholar] [CrossRef] [Green Version]
- Mejri, S.; Siah, A.; Coutte, F.; Magnin-Robert, M.; Randoux, B.; Tisserant, B.; Krier, F.; Jacques, P.; Reignault, P.; Halama, P. Biocontrol of the wheat pathogen Zymoseptoria tritici using cyclic lipopeptides from Bacillus subtilis. Environ. Sci. Pollut. Res. 2018, 25, 29822–29833. [Google Scholar] [CrossRef]
- Gong, A.-D.; Li, H.-P.; Yuan, Q.-S.; Song, X.-S.; Yao, W.; He, W.-J.; Zhang, J.-B.; Liao, Y.-C. Antagonistic mechanism of iturin A and plipastatin A from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef] [Green Version]
- Jauregi, P.; Kourmentza, K. Chapter 3—Membrane Filtration of Biosurfactants. In Separation of Functional Molecules in Food by Membrane Technology; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 79–112. [Google Scholar]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef] [Green Version]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef] [Green Version]
- Sha, R.; Jiang, L.; Meng, Q.; Zhang, G.; Song, Z. Producing cell-free culture broth of rhamnolipids as a cost-effective fungicide against plant pathogens. J. Basic Microbiol. 2012, 52, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Vatsa, P.; Sanchez, L.; Clement, C.; Baillieul, F.; Dorey, S. Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. Int. J. Mol. Sci. 2010, 11, 5095–5108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- De Britto, D.; Campana-Filho, S.P. Kinetics of the thermal degradation of chitosan. Thermochim. Acta 2007, 465, 73–82. [Google Scholar] [CrossRef]
- Romano, P.; Fabritius, H.; Raabe, D. The exoskeleton of the lobster Homarus americanus as an example of a smart anisotropic biological material. Acta Biomater. 2007, 3, 301–309. [Google Scholar] [CrossRef]
- Platel, R.; Chaveriat, L.; Le Guenic, S.; Pipeleers, R.; Magnin-Robert, M.; Randoux, B.; Trapet, P.; Lequart, V.; Joly, N.; Halama, P.; et al. Importance of the C12 carbon chain in the biological activity of rhamnolipids conferring protection in wheat against Zymoseptoria tritici. Molecules 2020, 26, 40. [Google Scholar] [CrossRef]
- Robineau, M.; Le Guenic, S.; Sanchez, L.; Chaveriat, L.; Lequart, V.; Joly, N.; Calonne, M.; Jacquard, C.; Declerck, S.; Martin, P.; et al. Synthetic mono-rhamnolipids display direct antifungal effects and trigger an innate immune response in tomato against Botrytis cinerea. Molecules 2020, 25, 3108. [Google Scholar] [CrossRef]
- Monnier, N.; Furlan, A.L.; Buchoux, S.; Deleu, M.; Dauchez, M.; Rippa, S.; Sarazin, C. Exploring the dual interaction of natural rhamnolipids with plant and fungal biomimetic plasma membranes through biophysical studies. Int. J. Mol. Sci. 2019, 20, 1009. [Google Scholar] [CrossRef] [Green Version]
- Deravel, J.; Lemière, S.; Coutte, F.; Krier, F.; Hese, N.V.; Béchet, M.; Sourdeau, N.; Höfte, M.; Leprêtre, A.; Jacques, P. Mycosubtilin and surfactin are efficient, low ecotoxicity molecules for the biocontrol of lettuce downy mildew. Appl. Microbiol. Biotechnol. 2014, 10, 6255–6264. [Google Scholar] [CrossRef]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef] [Green Version]
- Henry, G.; Deleu, M.; Jourdan, E.; Thonart, P.; Ongena, M. The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune-related defence responses. Cell. Microbiol. 2011, 13, 1824–1837. [Google Scholar] [CrossRef] [PubMed]
- Cawoy, H.; Mariutto, M.; Henry, G.; Fisher, C.; Vasilyeva, N.; Thonart, P.; Dommes, J.; Ongena, M. Plant defense stimulation by natural isolates of Bacillus depends on efficient surfactin production. Mol. Plant-Microbe Interact. 2014, 27, 87–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoll, A.; Salvatierra-Martínez, R.; González, M.; Araya, M. The role of surfactin production by Bacillus velezensis on colonization, biofilm formation on tomato root and leaf surfaces and subsequent protection (ISR) against Botrytis cinerea. Microorganisms 2021, 9, 2251. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Héloir, M.-C.; Zhang, X.; Geissler, M.; Trouvelot, S.; Jacquens, L.; Henkel, M.; Su, X.; Fang, X.; Wang, Q.; et al. Surfactin and fengycin contribute to the protection of a Bacillus subtilis strain against grape downy mildew by both direct effect and defence stimulation. Mol. Plant Pathol. 2019, 20, 1037–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, J.; Tonelli, M.L.; Figueredo, M.S.; Ibáñez, F.; Fabra, A. The lipopeptide surfactin triggers induced systemic resistance and priming state responses in Arachis hypogaea L. Eur. J. Plant Pathol. 2018, 152, 845–851. [Google Scholar] [CrossRef]
- Le Mire, G.; Siah, A.; Brisset, M.-N.; Gaucher, M.; Deleu, M.; Jijakli, M.H. Surfactin protects wheat against Zymoseptoria tritici and activates both salicylic acid- and jasmonic acid-dependent defense responses. Agriculture 2018, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Waewthongrak, W.; Leelasuphakul, W.; McCollum, G. Cyclic lipopeptides from Bacillus subtilis ABS–S14 elicit defense-related gene expression in citrus fruit. PLoS ONE 2014, 9, e109386. [Google Scholar] [CrossRef]
- Farace, G.; Fernandez, O.; Jacquens, L.; Coutte, F.; Krier, F.; Jacques, P.; Clément, C.; Barka, E.A.; Jacquard, C.; Dorey, S. Cyclic lipopeptides from Bacillus subtilis activate distinct patterns of defence responses in grapevine: Cyclic lipopeptides in grapevine innate immunity. Mol. Plant Pathol. 2015, 16, 177–187. [Google Scholar] [CrossRef]
- Aziz, A.; Trotel-Aziz, P.; Dhuicq, L.; Jeandet, P.; Couderchet, M.; Vernet, G. Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology 2006, 96, 1188–1194. [Google Scholar] [CrossRef] [Green Version]
- Benhamou, N.; Lafontaine, P.J.; Nicole, M. Induction of systemic resistance to Fusarium crown and root rot in tomato plants by seed treatment with chitosan. Phytopathology 1994, 84, 1432–1444. [Google Scholar] [CrossRef]
- Faoro, F.; Maffi, D.; Cantu, D.; Iriti, M. Chemical-induced resistance against powdery mildew in barley: The effects of chitosan and benzothiadiazole. BioControl 2008, 53, 387–401. [Google Scholar] [CrossRef]
- Bhaskara Reddy, M.V.; Arul, J.; Angers, P.; Couture, L. Chitosan treatment of wheat seeds induces resistance to Fusarium graminearum and improves seed quality. J. Agric. Food Chem. 1999, 47, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Khawar, K.M.; Camara, M.C.; Carvalho, L.B.; Fraceto, L.F.; Morsi, R.E.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Ullah, H.; et al. Chitosan-based delivery systems for plants: A brief overview of recent advances and future directions. Int. J. Biol. Macromol. 2020, 154, 683–697. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Rocha, M.A.M.; Coimbra, M.A.; Nunes, C. Applications of chitosan and their derivatives in beverages: A critical review. Sens. Sci. Consum. Percept.-Food Phys. Mater. Sci. 2017, 15, 61–69. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G.H. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vander, P.; Vrum, K.M.; Domard, A.; Eddine El Gueddari, N.; Moerschbacher, B.M. Comparison of the ability of partially N-acetylated chitosans and chitooligosaccharides to elicit resistance reactions in wheat leaves. Plant Physiol. 1998, 118, 1353–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Tian, S.; Meng, X.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Jayaraj, J.; Rahman, M.; Wan, A.; Punja, Z.K. Enhanced resistance to foliar fungal pathogens in carrot by application of elicitors. Ann. Appl. Biol. 2009, 155, 71–80. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Chen, C.-Y. Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour. Technol. 2008, 99, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Park, R.-D.; Jo, K.-J.; Jo, Y.; Jin, Y.-L.; Kim, K.-Y.; Shim, J.-H. Variation of antifungal activities of chitosans on plant pathogens. J. Microbiol. Biotechnol. 2002, 12, 84–88. [Google Scholar]
- Aranaz, I.; Harris, R.; Heras, A. Chitosan amphiphilic derivatives. Chemistry and applications. Curr. Org. Chem. 2010, 14, 308–330. [Google Scholar] [CrossRef]
- Cabrera, J.C.; Van Cutsem, P. Preparation of chitooligosaccharides with degree of polymerization higher than 6 by acid or enzymatic degradation of chitosan. Biochem. Eng. J. 2005, 25, 165–172. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and interfacial properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef] [Green Version]
- Reignault, P.; Walters, D. Topical induction of inducers for disease control. In Induced Resistance for Plant Defence: A Sustainable Approach to Crop Protection; Walters, D., Newton, A., Lyon, G., Walters, D., Newton, A., Lyon, G., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 179–200. [Google Scholar]
- Marangon, C.A.; Martins, V.C.A.; Ling, M.H.; Melo, C.C.; Plepis, A.M.G.; Meyer, R.L.; Nitschke, M. Combination of rhamnolipid and chitosan in nanoparticles boosts their antimicrobial efficacy. ACS Appl. Mater. Interfaces 2020, 12, 5488–5499. [Google Scholar] [CrossRef]
- Varnier, A.-L.; Sanchez, L.; Vatsa, P.; Boudesocque, L.; Garcia-Brugger, A.; Rabenoelina, F.; Sorokin, A.; Renault, J.-H.; Kauffmann, S.; Pugin, A.; et al. Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant Cell Environ. 2009, 32, 178–193. [Google Scholar] [CrossRef]
- Domszy, J.G.; Roberts, G.A.F. Evaluation of infrared spectroscopic techniques for analysing chitosan. Makromol. Chem. 1985, 186, 1671–1677. [Google Scholar] [CrossRef]
- Kourmentza, K.; Gromada, X.; Michael, N.; Degraeve, C.; Vanier, G.; Ravallec, R.; Coutte, F.; Karatzas, K.A.; Jauregi, P. Antimicrobial activity of lipopeptide biosurfactants against foodborne pathogen and food spoilage microorganisms and their cytotoxicity. Front. Microbiol. 2021, 11, 561060. [Google Scholar] [CrossRef] [PubMed]
- Ongena, M.; Jourdan, E.; Adam, A.; Paquot, M.; Brans, A.; Joris, B.; Arpigny, J.-L.; Thonart, P. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 2007, 9, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Coutte, F.; Leclère, V.; Béchet, M.; Guez, J.-S.; Lecouturier, D.; Chollet-Imbert, M.; Dhulster, P.; Jacques, P. Effect of Pps disruption and constitutive expression of SrfA on surfactin productivity, spreading and antagonistic properties of Bacillus subtilis 168 derivatives. J. Appl. Microbiol. 2010, 109, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Randoux, B.; Renard, D.; Nowak, E.; Sanssené, J.; Courtois, J.; Durand, R.; Reignault, P. Inhibition of Blumeria graminis f.sp. tritici germination and partial enhancement of wheat defenses by Milsana®. Phytopathology 2006, 96, 1278–1286. [Google Scholar] [CrossRef] [Green Version]
- Tayeh, C.; Randoux, B.; Tisserant, B.; Khong, G.; Jacques, P.; Reignault, P. Are ineffective defence reactions potential target for induced resistance during the compatible wheat-powdery mildew interaction? Plant Physiol. Biochem. 2015, 96, 9–19. [Google Scholar] [CrossRef]
- Mustafa, G.; Randoux, B.; Tisserant, B.; Fontaine, J.; Magnin-Robert, M.; Lounès-Hadj Sahraoui, A.; Reignault, P. Phosphorus supply, arbuscular mycorrhizal fungal species, and plant genotype impact on the protective efficacy of mycorrhizal inoculation against wheat powdery mildew. Mycorrhiza 2016, 26, 685–697. [Google Scholar] [CrossRef]






| m/z | Types | Ion Composition |
|---|---|---|
| 524.2 | [M + Na]+ | (GlcN)3 |
| 540.2 | [M + K]+ | |
| 685.3 | [M + Na]+ | (GlcN)4 |
| 701.3 | [M + K]+ | |
| 846.3 | [M + Na]+ | (GlcN)5 |
| 862.3 | [M + K]+ | |
| 1007.4 | [M + Na]+ | (GlcN)6 |
| 1023.4 | [M + K]+ | |
| 1168.5 | [M + Na]+ | (GlcN)7 |
| 1184.5 | [M + K]+ | |
| 1329.6 | [M + Na]+ | (GlcN)8 |
| 1345.5 | [M + K]+ | |
| 1490.6 | [M + Na]+ | (GlcN)9 |
| 1506.6 | [M + K]+ | |
| 1651.7 | [M + Na]+ | (GlcN)10 |
| 1667.7 | [M + K]+ | |
| 1812.8 | [M + Na]+ | (GlcN)11 |
| 1828.8 | [M + K]+ | |
| 1973.8 | [M + Na]+ | (GlcN)12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raouani, N.E.H.; Claverie, E.; Randoux, B.; Chaveriat, L.; Yaseen, Y.; Yada, B.; Martin, P.; Cabrera, J.C.; Jacques, P.; Reignault, P.; et al. Bio-Inspired Rhamnolipids, Cyclic Lipopeptides and a Chito-Oligosaccharide Confer Protection against Wheat Powdery Mildew and Inhibit Conidia Germination. Molecules 2022, 27, 6672. https://doi.org/10.3390/molecules27196672
Raouani NEH, Claverie E, Randoux B, Chaveriat L, Yaseen Y, Yada B, Martin P, Cabrera JC, Jacques P, Reignault P, et al. Bio-Inspired Rhamnolipids, Cyclic Lipopeptides and a Chito-Oligosaccharide Confer Protection against Wheat Powdery Mildew and Inhibit Conidia Germination. Molecules. 2022; 27(19):6672. https://doi.org/10.3390/molecules27196672
Chicago/Turabian StyleRaouani, Nour El Houda, Elodie Claverie, Béatrice Randoux, Ludovic Chaveriat, Yazen Yaseen, Bopha Yada, Patrick Martin, Juan Carlos Cabrera, Philippe Jacques, Philippe Reignault, and et al. 2022. "Bio-Inspired Rhamnolipids, Cyclic Lipopeptides and a Chito-Oligosaccharide Confer Protection against Wheat Powdery Mildew and Inhibit Conidia Germination" Molecules 27, no. 19: 6672. https://doi.org/10.3390/molecules27196672