Constructing a Visible-Active CoFe2O4@Bi2O3/NiO Nanoheterojunction as Magnetically Recoverable Photocatalyst with Boosted Ofloxacin Degradation Efficiency
Abstract
:1. Introduction
2. Results
2.1. Structural Analysis of Prepared Catalysts
2.2. Microstructural and FTIR Analysis
2.3. XPS Analysis
2.4. Raman Spectroscopy and Magnetic Studies
2.5. Complete Band Structure Determination
2.6. Degradation Performance against Ofloxacin
Operational Parameters
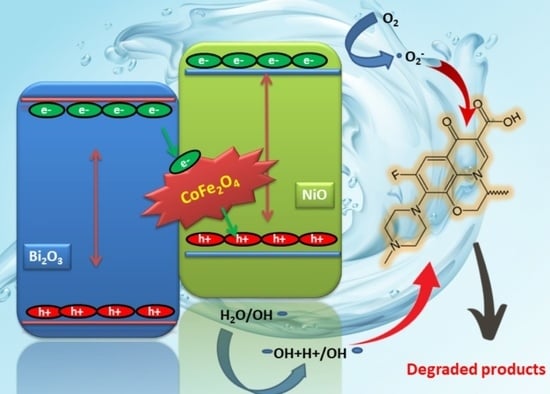
2.7. Pollutant Degradation Pathway and Photocatalytic Mechanism
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Bi2O3, NiO and CoFe2O4 Nanoparticles
3.3. Synthesis of CoFe2O4@Bi2O3/NiO Heterojunction
3.4. Characterization Details of Catalysts
3.5. Photocatalytic Degradation Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mudhoo, A.; Paliya, S.; Goswami, P.; Singh, M.; Lofrano, G.; Carotenuto, M.; Carraturo, F.; Libralato, G.; Guida, M.; Usman, M.; et al. Fabrication, functionalization and performance of doped photocatalysts for dye degradation and mineralization: A review. Environ. Chem. Lett. 2020, 18, 1825–1903. [Google Scholar] [CrossRef]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of carbon dots in environmental pollution control: A review. Chem. Eng. J. 2021, 406, 126848. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, Z.; Wei, J.; Xu, Q.; Zhang, X.; Wei, Z. Efficient degradation of antibiotics by photo-Fenton reactive ceramic membrane with high flux by a facile spraying method under visible LED light. J. Clean. Prod. 2022, 366, 132849. [Google Scholar] [CrossRef]
- Su, Q.; Li, J.; Yuan, H.; Wang, B.; Wang, Y.; Li, Y.; Xing, Y. Visible-light-driven photocatalytic degradation of ofloxacin by g-C3N4/NH2-MIL-88B(Fe) heterostructure: Mechanisms, DFT calculation, degradation pathway and toxicity evolution. Chem. Eng. J. 2022, 427, 131594. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Gao, P.; Tian, X.; Nie, Y.; Yang, C.; Zhou, Z.; Wang, Y. Promoted peroxymonosulfate activation into singlet oxygen over perovskite for ofloxacin degradation by controlling the oxygen defect concentration. Chem. Eng. J. 2019, 359, 828–839. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Kumar, P.S.; Alodhayb, A.; Alothman, Z.A.; Dhiman, P.; Stadler, F.J. Carbon quantum dots embedded trimetallic oxide: Characterization and photocatalytic degradation of Ofloxacin. J. Water Process Eng. 2022, 48, 102853. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Cao, Z.; Xu, J.; Hu, J.; Huang, Y.; Cui, C.; Liu, H.; Wang, H. Simultaneous enhancements of light-harvesting and charge transfer in UiO-67/CdS/rGO composites toward ofloxacin photo-degradation. Chem. Eng. J. 2020, 381, 122771. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Fan, W.-Y.; Yao, M.-C.; Yuan, L.; Sheng, G.-P. Enhanced Photodegradation of Extracellular Antibiotic Resistance Genes by Dissolved Organic Matter Photosensitization. Environ. Sci. Technol. 2019, 53, 10732–10740. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Al-Muhtaseb, A.a.H.; García-Peñas, A.; Mola, G.T.; Si, C.; Stadler, F.J. Bio-inspired and biomaterials-based hybrid photocatalysts for environmental detoxification: A review. Chem. Eng. J. 2020, 382, 122937. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone antibiotics: An emerging class of environmental micropollutants. Sci. Total Environ. 2014, 500–501, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kumar, A.; Sharma, G.; Naushad, M.; Alberto, G.-P.; Stadler, F.J. A dual-functional integrated Ni5P4/g-C3N4 S-scheme heterojunction for high performance synchronous photocatalytic hydrogen evolution and multi-contaminant removal with a waste-to-energy conversion. J. Mol. Liq. 2022, 366, 120147. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Kumari, A.; Guo, C.; Naushad, M.; Vo, D.-V.N.; Iqbal, J.; Stadler, F.J. Construction of dual Z-scheme g-C3N4/Bi4Ti3O12/Bi4O5I2 heterojunction for visible and solar powered coupled photocatalytic antibiotic degradation and hydrogen production: Boosting via I-/I3- and Bi3+/Bi5+ redox mediators. Appl. Catal. B Environ. 2020, 284, 119808. [Google Scholar] [CrossRef]
- Dhiman, P.; Goyal, D.; Rana, G.; Kumar, A.; Sharma, G.; Linxin; Kumar, G. Recent advances on carbon-based nanomaterials supported single-atom photo-catalysts for waste water remediation. J. Nanostructure Chem. 2022. [Google Scholar] [CrossRef]
- Dhiman, P.; Rana, G.; Kumar, A.; Sharma, G.; Vo, D.-V.N.; Naushad, M. ZnO-based heterostructures as photocatalysts for hydrogen generation and depollution: A review. Environ. Chem. Lett. 2022, 20, 1047–1081. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Tian, X.; Hong, Y.; Nie, Y.; Su, N.; Jin, G.; Zhai, Z.; Fu, C. Highly efficient photocatalytic degradation of oil pollutants by oxygen deficient SnO2 quantum dots for water remediation. Chem. Eng. J. 2021, 404, 127146. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, L.; Li, C.; Zhang, T.; Yan, T.; Yu, J.; Jiang, X.; Jiao, F. Construction of diatomite/ZnFe layered double hydroxides hybrid composites for enhanced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. A Chem. 2018, 367, 302–311. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Xie, Y.; Liang, Y.; Gong, X.; Qin, P.; Jiang, L.; Guo, J.; Liu, C.; Wu, Z. Principles, synthesis and applications of dual Z-scheme photocatalysts. Coord. Chem. Rev. 2022, 467, 214596. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.; Jaroniec, M. All-Solid-State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef]
- Yang, L.; Han, Q.; Wang, X.; Zhu, J. Highly efficient removal of aqueous chromate and organic dyes by ultralong HCOOBiO nanowires. Chem. Eng. J. 2015, 262, 169–178. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Yang, Y.; Rodriguez, R.D.; Lipovka, A.; Lu, Y.; Huang, H.; Chen, J. Ag nanoparticle-decorated Bi2O3-TiO2 heterogeneous nanotubular photocatalysts for enhanced degradation of organic contaminants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129233. [Google Scholar] [CrossRef]
- Sang, Y.; Cao, X.; Dai, G.; Wang, L.; Peng, Y.; Geng, B. Facile one-pot synthesis of novel hierarchical Bi2O3/Bi2S3 nanoflower photocatalyst with intrinsic p-n junction for efficient photocatalytic removals of RhB and Cr(VI). J. Hazard. Mater. 2020, 381, 120942. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.; Chen, C.; Liu, J.; Zou, S.; Xiao, L.; Fan, J. Selectively depositing Bi2O3 quantum dots on TiO2 nanotubes for efficient visible-light-driven photocatalysis. Mater. Lett. 2021, 288, 129413. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Yang, Y.; Zhou, Z.; Shang, Y.; Zhuang, X. Photocatalytic degradation of sulfonamides by Bi2O3-TiO2/PAC ternary composite: Mechanism, degradation pathway. J. Water Process Eng. 2020, 36, 101335. [Google Scholar] [CrossRef]
- Fu, F.; Shen, H.; Xue, W.; Zhen, Y.; Soomro, R.A.; Yang, X.; Wang, D.; Xu, B.; Chi, R. Alkali-assisted synthesis of direct Z-scheme based Bi2O3/Bi2MoO6 photocatalyst for highly efficient photocatalytic degradation of phenol and hydrogen evolution reaction. J. Catal. 2019, 375, 399–409. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Wang, G.; Fang, H.; Yuan, X.; Lu, C. Photocatalytic removal of metronidazole and Cr (Ⅵ) by a novel Zn3In2S6/Bi2O3 S-scheme heterojunction: Performance, mechanism insight and toxicity assessment. Chem. Eng. J. 2022, 450, 138167. [Google Scholar] [CrossRef]
- Zhao, Y.; Dang, P.; Gao, Y.; Li, Y.; Xie, H.; Yang, C. Double Z-scheme Co3O4/Bi4O7/Bi2O3 composite activated peroxymonosulfate to efficiently degrade tetracycline under visible light. Environ. Sci. Pollut. Res. 2022, 29, 79184–79198. [Google Scholar] [CrossRef]
- Yasin, M.; Saeed, M.; Muneer, M.; Usman, M.; ul Haq, A.; Sadia, M.; Altaf, M. Development of Bi2O3-ZnO heterostructure for enhanced photodegradation of rhodamine B and reactive yellow dyes. Surf. Interfaces 2022, 30, 101846. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Wang, Y.; Xin, C.; Zhang, P.; Liu, D.; Mamba, B.B.; Kefeni, K.K.; Kuvarega, A.T.; Gui, J. Hollow β-Bi2O3@CeO2 heterostructure microsphere with controllable crystal phase for efficient photocatalysis. Chem. Eng. J. 2020, 387, 124100. [Google Scholar] [CrossRef]
- Goud, B.S.; Koyyada, G.; Jung, J.H.; Reddy, G.R.; Shim, J.; Nam, N.D.; Vattikuti, S.V.P. Surface oxygen vacancy facilitated Z-scheme MoS2/Bi2O3 heterojunction for enhanced visible-light driven photocatalysis-pollutant degradation and hydrogen production. Int. J. Hydrogen Energy 2020, 45, 18961–18975. [Google Scholar] [CrossRef]
- Ghasemi, I.; Haghighi, M.; Talati, A.; Abbasi Asl, E. Facile sono-design of 3D flower-like NiO–CuFe2O4 nano-heterostructure as an efficient and magnetically separable catalyst for photodegradation of organic dyes. J. Clean. Prod. 2022, 335, 130355. [Google Scholar] [CrossRef]
- Lahiri, S.K.; Zhang, C.; Sillanpää, M.; Liu, L. Nanoporous NiO@SiO2 photo-catalyst prepared by ion-exchange method for fast elimination of reactive dyes from wastewater. Mater. Today Chem. 2022, 23, 100677. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, Y.; Jiang, Z.; Xu, L.; Liu, C. Fabrication and Characterization of a Novel Composite Magnetic Photocatalyst β-Bi2O3/BiVO4/MnxZn1−xFe2O4 for Rhodamine B Degradation under Visible Light. Nanomaterials 2020, 10, 797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarusheh, H.S.; Yusuf, A.; Banat, F.; Haija, M.A.; Palmisano, G. Integrated photocatalytic technologies in water treatment using ferrites nanoparticles. J. Environ. Chem. Eng. 2022, 10, 108204. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mengelizadeh, N.; Kassim, W.M.S.; Sillanpää, M.; Siddiqui, S.H.; Shahbaksh, S.; Balarak, D. Sonophotocatalytic degradation and operational parameters optimization of diazinon using magnetic cobalt–graphene nanocomposite as a catalyst. J. Water Process Eng. 2022, 46, 102548. [Google Scholar] [CrossRef]
- Mmelesi, O.K.; Masunga, N.; Kuvarega, A.; Nkambule, T.T.I.; Mamba, B.B.; Kefeni, K.K. Cobalt ferrite nanoparticles and nanocomposites: Photocatalytic, antimicrobial activity and toxicity in water treatment. Mater. Sci. Semicond. Process. 2021, 123, 105523. [Google Scholar] [CrossRef]
- Al-Kahtani, A.A.; Abou Taleb, M.F. Photocatalytic degradation of Maxilon C.I. basic dye using CS/CoFe2O4/GONCs as a heterogeneous photo-Fenton catalyst prepared by gamma irradiation. J. Hazard. Mater. 2016, 309, 10–19. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H.; Rakass, S.; Al Wadaani, F.T.; Al-ghamdi, K.J.; Omer, A.; Messali, M.; Abboudi, M. Synthesis, characterization and photocatalytic activity of α-Bi2O3 nanoparticles. J. Taibah Univ. Sci. 2015, 9, 508–512. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Qiao, H.; Yang, H.; Zhang, C.; Yan, X. Characterization of NiO nanoparticles by anodic arc plasma method. J. Alloys Compd. 2009, 479, 855–858. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Al-Muhtaseb, A.a.H.; Naushad, M.; Ghfar, A.A.; Guo, C.; Stadler, F.J. Biochar-templated g-C3N4/Bi2O2CO3/CoFe2O4 nano-assembly for visible and solar assisted photo-degradation of paraquat, nitrophenol reduction and CO2 conversion. Chem. Eng. J. 2018, 339, 393–410. [Google Scholar] [CrossRef]
- Thirumurthy, K.; Thirunarayanan, G. A facile designed highly moderate craspedia flowerlike sulphated Bi2O3-fly ash catalyst: Green synthetic strategy for (6H-pyrido[3,2-b]carbazol-4-yl)aniline derivatives in water. Arab. J. Chem. 2018, 11, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Bhogal, S.; Gupta, V.K.; Agarwal, S.; Kumar, A.; Pathania, D.; Mola, G.T.; Stadler, F.J. Algal biochar reinforced trimetallic nanocomposite as adsorptional/photocatalyst for remediation of malachite green from aqueous medium. J. Mol. Liq. 2019, 275, 499–509. [Google Scholar] [CrossRef]
- Singh, M.; Dosanjh, H.S.; Singh, H. Surface modified spinel cobalt ferrite nanoparticles for cationic dye removal: Kinetics and thermodynamics studies. J. Water Process Eng. 2016, 11, 152–161. [Google Scholar] [CrossRef]
- Elamin, N.Y.; Indumathi, T.; Kumar, E.R. Murraya koenigii mediated synthesis of cobalt doped NiO nanoparticles: Evaluation of structural, optical properties and anti-bacterial activity. Phys. E Low-Dimens. Syst. Nanostructures 2022, 142, 115295. [Google Scholar] [CrossRef]
- Singh, S.; Sahoo, R.K.; Shinde, N.M.; Yun, J.M.; Mane, R.S.; Kim, K.H. Synthesis of Bi2O3-MnO2 nanocomposite electrode for wide-potential window high performance supercapacitor. Energies 2019, 12, 3320. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.Q.; Ahmad, K.; Alsalme, A.; Kim, H. Hydrothermal synthesis of nanostructured NiO for hydrazine sensing application. Mater. Chem. Phys. 2022, 289, 126463. [Google Scholar] [CrossRef]
- Cárdenas-Arenas, A.; Bailón-García, E.; Lozano-Castelló, D.; Da Costa, P.; Bueno-López, A. Stable NiO–CeO2 nanoparticles with improved carbon resistance for methane dry reforming. J. Rare Earths 2022, 40, 57–62. [Google Scholar] [CrossRef]
- Dhiman, P.; Naushad, M.; Batoo, K.M.; Kumar, A.; Sharma, G.; Ghfar, A.A.; Kumar, G.; Singh, M. Nano FexZn1−xO as a tuneable and efficient photocatalyst for solar powered degradation of bisphenol A from aqueous environment. J. Clean. Prod. 2017, 165, 1542–1556. [Google Scholar] [CrossRef]
- Kumar, Y.; Sharma, A.; Ahmed, M.A.; Mali, S.S.; Hong, C.K.; Shirage, P.M. Morphology-controlled synthesis and enhanced energy product (BH)max of CoFe2O4 nanoparticles. New J. Chem. 2018, 42, 15793–15802. [Google Scholar] [CrossRef]
- Vivier, V.; Régis, A.; Sagon, G.; Nedelec, J.Y.; Yu, L.T.; Cachet-Vivier, C. Cyclic voltammetry study of bismuth oxide Bi2O3 powder by means of a cavity microelectrode coupled with Raman microspectrometry. Electrochim. Acta 2001, 46, 907–914. [Google Scholar] [CrossRef]
- Vila, M.; Díaz-Guerra, C.; Piqueras, J. Luminescence and Raman study of α-Bi2O3 ceramics. Mater. Chem. Phys. 2012, 133, 559–564. [Google Scholar] [CrossRef]
- Liu, X.; Cao, H.; Yin, J. Generation and photocatalytic activities of Bi@Bi2O3 microspheres. Nano Res. 2011, 4, 470–482. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Sildos, I.; Pärs, M. Polarisation dependent Raman study of single-crystal nickel oxide. Cent. Eur. J. Phys. 2011, 9, 1096–1099. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Sildos, I.; Puust, L.; Grabis, J. Magnon and phonon excitations in nanosized NiO. Latv. J. Phys. Tech. Sci. 2019, 56, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Yan, M.; Chen, Q.-G.; Fan, C.-M.; Zhou, H.-Y.; Xu, A.-W. Novel one-dimensional Bi2O3–Bi2WO6 p–n hierarchical heterojunction with enhanced photocatalytic activity. J. Mater. Chem. A 2014, 2, 8517–8524. [Google Scholar] [CrossRef]
- Hou, J.; Yang, C.; Wang, Z.; Zhou, W.; Jiao, S.; Zhu, H. In situ synthesis of α–β phase heterojunction on Bi2O3 nanowires with exceptional visible-light photocatalytic performance. Appl. Catal. B Environ. 2013, 142–143, 504–511. [Google Scholar] [CrossRef] [Green Version]
- Kirankumar, V.S.; Sumathi, S. Photocatalytic and antibacterial activity of bismuth and copper co-doped cobalt ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 8738–8746. [Google Scholar] [CrossRef]
- Prathapani, S.; Bhargava, P.; Mallick, S. Electronic band structure and carrier concentration of formamidinium–cesium mixed cation lead mixed halide hybrid perovskites. Appl. Phys. Lett. 2018, 112, 092104. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.K.; Sharma, G.; Guo, C.; Vo, D.-V.N.; Iqbal, J.; Naushad, M.; Stadler, F.J. Silicate glass matrix@Cu2O/Cu2V2O7 p-n heterojunction for enhanced visible light photo-degradation of sulfamethoxazole: High charge separation and interfacial transfer. J. Hazard. Mater. 2021, 402, 123790. [Google Scholar] [CrossRef]
- Liang, L.; Gao, S.; Zhu, J.; Wang, L.; Xiong, Y.; Xia, X.; Yang, L. The enhanced photocatalytic performance toward carbamazepine by nitrogen-doped carbon dots decorated on BiOBr/CeO2: Mechanism insight and degradation pathways. Chem. Eng. J. 2020, 391, 123599. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, N.; Chauhan, R.; Singh, V.; Srivastava, V.C.; Bhatnagar, R. Growth of hierarchical ZnO nano flower on large functionalized rGO sheet for superior photocatalytic mineralization of antibiotic. Chem. Eng. J. 2020, 392, 123746. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, J.; Ji, H.; Li, S.; Chen, L.; Huang, T.; Xu, C.; Chen, X.; Liu, W. Photocatalytic degradation of ofloxacin by perovskite-type NaNbO3 nanorods modified g-C3N4 heterojunction under simulated solar light: Theoretical calculation, ofloxacin degradation pathways and toxicity evolution. Chem. Eng. J. 2020, 400, 125918. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Chen, M. Novel Z-scheme LaVO4/Bi3O4Cl heterojunctions for highly efficient degradation of ofloxacin under visible light irradiation. J. Alloys Compd. 2022, 925, 166653. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Ahamad, T.; Veses, R.C.; Stadler, F.J. Highly visible active Ag2CrO4/Ag/BiFeO3@RGO nano-junction for photoreduction of CO2 and photocatalytic removal of ciprofloxacin and bromate ions: The triggering effect of Ag and RGO. Chem. Eng. J. 2019, 370, 148–165. [Google Scholar] [CrossRef]
- Dhiman, P.; Patial, M.; Kumar, A.; Alam, M.; Naushad, M.; Sharma, G.; Vo, D.-V.N.; Kumar, R. Environmental friendly and robust Mg0.5−xCuxZn0.5Fe2O4 spinel nanoparticles for visible light driven degradation of Carbamazepine: Band shift driven by dopants. Mater. Lett. 2021, 284, 129005. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, A.; Guo, C.; Sharma, G.; Katubi, K.M.M.; Alzahrani, F.M.; Naushad, M.; Sillanpää, M.; Dhiman, P.; Stadler, F.J. Acceleration of photo-reduction and oxidation capabilities of Bi4O5I2/SPION@calcium alginate by metallic Ag: Wide spectral removal of nitrate and azithromycin. Chem. Eng. J. 2021, 423, 130173. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhiman, P.; Sharma, G.; Alodhayb, A.N.; Kumar, A.; Rana, G.; Sithole, T.; ALOthman, Z.A. Constructing a Visible-Active CoFe2O4@Bi2O3/NiO Nanoheterojunction as Magnetically Recoverable Photocatalyst with Boosted Ofloxacin Degradation Efficiency. Molecules 2022, 27, 8234. https://doi.org/10.3390/molecules27238234
Dhiman P, Sharma G, Alodhayb AN, Kumar A, Rana G, Sithole T, ALOthman ZA. Constructing a Visible-Active CoFe2O4@Bi2O3/NiO Nanoheterojunction as Magnetically Recoverable Photocatalyst with Boosted Ofloxacin Degradation Efficiency. Molecules. 2022; 27(23):8234. https://doi.org/10.3390/molecules27238234
Chicago/Turabian StyleDhiman, Pooja, Gaurav Sharma, Abdullah N. Alodhayb, Amit Kumar, Garima Rana, Thandiwe Sithole, and Zeid A. ALOthman. 2022. "Constructing a Visible-Active CoFe2O4@Bi2O3/NiO Nanoheterojunction as Magnetically Recoverable Photocatalyst with Boosted Ofloxacin Degradation Efficiency" Molecules 27, no. 23: 8234. https://doi.org/10.3390/molecules27238234