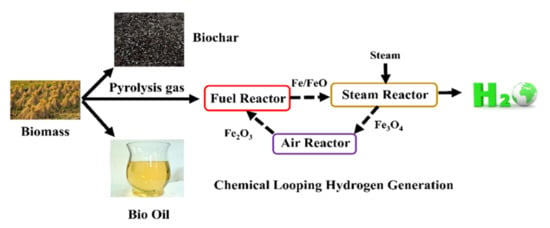
New Process Combining Fe-Based Chemical Looping and Biomass Pyrolysis for Cogeneration of Hydrogen, Biochar, Bio-Oil and Electricity with In-Suit CO2 Separation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material and Energy Calculation of the Proposed System
2.2. Exergy Flowchart
2.3. Effect of Pyrolysis Temperature
2.4. Effect of Fuel Reactor Operation Temperature
2.5. Effect of Steam Reactor Temperature
2.6. Effect of Air Reactor Operating Temperature
3. Methods and Materials
3.1. Materials
3.2. Model Description
3.2.1. Biomass Pyrolysis Unit
3.2.2. Chemical Looping Hydrogen Generation Unit
3.3. Evaluation Index of the Proposed System
3.4. Thermodynamic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pashchenko, D. Liquid organic hydrogen carriers (LOHCs) in the thermochemical waste heat recuperation systems: The energy and mass balances. Int. J. Hydrog. Energy 2022, 47, 28721–28729. [Google Scholar] [CrossRef]
- Pashchenko, D.; Mustafin, R.; Karpilov, I. Thermochemical recuperation by steam methane reforming as an efficient alternative to steam injection in the gas turbines. Energy 2022, 258, 124913. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Ma, J.; Wang, Z.; Liu, J.; Gong, X. ORR and OER of Co-N codoped carbon-based electrocatalysts enhanced by boundary layer oxygen molecules transfer. Carbon 2021, 172, 556–568. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.; Guo, S.; Li, J.; Yu, Z.; Song, S.; Li, J.; Fang, Y. High quality syngas production from pressurized K2CO3 catalytic coal gasification with in-situ CO2 capture. Int. J. Hydrog. Energy 2018, 43, 17091–17099. [Google Scholar] [CrossRef]
- Pashchenko, D. Experimental investigation of reforming and flow characteristics of a steam methane reformer filled with nickel catalyst of various shapes. Energy Convers. Manag. 2019, 185, 465–472. [Google Scholar] [CrossRef]
- Samprón, I.; de Diego, L.F.; García-Labiano, F.; Izquierdo, M.T.; Abad, A.; Adánez, J. Biomass Chemical Looping Gasification of pine wood using a synthetic Fe2O3/Al2O3 oxygen carrier in a continuous unit. Bioresour. Technol. 2020, 316, 123908. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, G.; Liu, F.; Li, N. Production of ethanol from corn straw based on chemical looping gasification: Economic analysis. Bioresour. Technol. 2022, 360, 127568. [Google Scholar] [CrossRef] [PubMed]
- Pashchenko, D. Experimental investigation of synthesis gas production by methane reforming with flue gas over a NiO-Al2O3 catalyst: Reforming characteristics and pressure drop. Int. J. Hydrog. Energy 2019, 44, 7073–7082. [Google Scholar] [CrossRef]
- Xu, D.; Yang, S.; Su, Y.; Shi, L.; Zhang, S.; Xiong, Y. Simultaneous production of aromatics-rich bio-oil and carbon nanomaterials from catalytic co-pyrolysis of biomass/plastic wastes and in-line catalytic upgrading of pyrolysis gas. Waste Manag. 2021, 121, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar]
- Zou, J.; Oladipo, J.; Fu, S.; Al-Rahbi, A.; Yang, H.; Wu, C.; Cai, N.; Williams, P.; Chen, H. Hydrogen production from cellulose catalytic gasification on CeO2/Fe2O3 catalyst. Energy Convers. Manag. 2018, 171, 241–248. [Google Scholar] [CrossRef]
- He, C.; Feng, X.; Chu, K.H. Process modeling and thermodynamic analysis of Lurgi fixed-bed coal gasifier in an SNG plant. Appl. Energy 2013, 111, 742–757. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Y.; Ma, D.; Liu, Q.; Yang, J. Process simulation of coal-direct chemical looping gasification for syngas production. RSC Adv. 2017, 7, 55450–55458. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Zeng, L.; Fan, L. Biomass direct chemical looping process: Process simulation. Fuel 2010, 89, 3773–3784. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, X.; Li, J.; Zhao, J.; Song, S.; Hao, Z.; Li, C.; Zhang, J.; Yu, Z.; Fang, Y. High-pressure rapid hydrogasification of pinewood for methane production using calcium looping concept. Energy Convers. Manag. 2020, 203, 112247. [Google Scholar] [CrossRef]
- Ma, S.; Chen, S.; Zhu, M.; Zhao, Z.; Hu, J.; Wu, M.; Toan, S.; Xiang, W. Enhanced sintering resistance of Fe2O3/CeO2 oxygen carrier for chemical looping hydrogen generation using core-shell structure. Int. J. Hydrog. Energy 2019, 44, 6491–6504. [Google Scholar] [CrossRef]
- Jia, L.; Cheng, P.; Yu, Y.; Chen, S.H.; Wang, C.X.; He, L.; Nie, H.T.; Wang, J.C.; Zhang, J.C.; Fan, B.G.; et al. Regeneration mechanism of a novel high-performance biochar mercury adsorbent directionally modified by multimetal multilayer loading. J. Environ. Manag. 2023, 326, 116790. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Clausen, L.R. Techno-economic analysis of polygeneration systems based on catalytic hydropyrolysis for the production of bio-oil and fuels. Energy Convers. Manag. 2019, 184, 539–558. [Google Scholar] [CrossRef]
- Situmorang, Y.A.; Zhao, Z.; An, P.; Yu, T.; Rizkiana, J.; Abudula, A.; Guan, G. A novel system of biomass-based hydrogen production by combining steam bio-oil reforming and chemical looping process. Appl. Energy 2020, 268, 115122. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, P.; Wang, J.; Wei, J.; Cao, P.; Bie, N.; Bai, Y.; Yu, G. Product characteristics of rice straw pyrolysis at different temperature: Role of inherent alkali and alkaline earth metals with different occurrence forms. J. Energy Inst. 2022, 101, 201–208. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, S. Multicomponent vaporization modeling of bio-oil and its mixtures with other fuels. Fuel 2012, 95, 471–480. [Google Scholar] [CrossRef]
- Zeng, D.; Cui, D.; Qiu, Y.; Li, M.; Ma, L.; Zhang, S.; Xiao, R. Mn-Fe-Al-O mixed spinel oxides as oxygen carrier for chemical looping hydrogen production with CO2 capture. Fuel 2020, 274, 117854. [Google Scholar] [CrossRef]
- Bock, S.; Stoppacher, B.; Malli, K.; Lammer, M.; Hacker, V. Techno-economic analysis of fixed-bed chemical looping for decentralized, fuel-cell-grade hydrogen production coupled with a 3 MWth biogas digester. Energy Convers. Manag. 2021, 250, 114801. [Google Scholar] [CrossRef]
- Boughanmi, H.; Lazaar, M.; Farhat, A.; Guizani, A. Evaluation of soil thermal potential under Tunisian climate using a new conic basket geothermal heat exchanger: Energy and exergy analysis. Appl. Therm. Eng. 2017, 113, 912–925. [Google Scholar] [CrossRef]
- Dai, B.; Zhang, L.; Cui, J.; Hoadley, A.; Zhang, L. Integration of pyrolysis and entrained-bed gasification for the production of chemicals from Victorian brown coal-Process simulation and exergy analysis. Fuel Process. Technol. 2017, 155, 21–31. [Google Scholar] [CrossRef]
- Cruz, P.L.; Iribarren, D.; Dufour, J. Exergy analysis of alternative configurations of a system coproducing synthetic fuels and electricity via biomass gasification, Fischer-Tropsch synthesis and a combined-cycle scheme. Fuel 2017, 194, 375–394. [Google Scholar] [CrossRef]
- Xiong, J.; Zhao, H.; Zheng, C. Exergy Analysis of a 600 MWe Oxy-combustion Pulverized-Coal-Fired Power Plant. Energy Fuels 2011, 25, 3854–3864. [Google Scholar] [CrossRef]
- Abuadala, A.; Dincer, I.; Naterer, G.F. Exergy analysis of hydrogen production from biomass gasification. Int. J. Hydrog. Energy 2010, 35, 4981–4990. [Google Scholar] [CrossRef]
- Tumen Ozdil, N.F.; Tantekin, A.; Erbay, Z. Energy and exergy analyses of a fluidized bed coal combustor steam plant in textile industry. Fuel 2016, 183, 441–448. [Google Scholar] [CrossRef]







| Components | Value (wt%) |
|---|---|
| Water | 28.11 |
| Hydroxyacetaldehyde | 25.32 |
| Hydroxypropanone | 14.12 |
| Propionic acid | 2.63 |
| Isoeugenol | 9.86 |
| Acetic acid | 15.37 |
| Phenol | 1.52 |
| Syringol | 0.75 |
| (5H)-furan-2-one | 2.32 |
| Stream | Component | Mass Flow (kg/h) | P (MPa) | T (°C) |
|---|---|---|---|---|
| 1 | Rice straw | 100 | 0.1 | 25 |
| 2 | Crushed rice straw | 100 | 0.1 | 25 |
| 3 | Biochar, bio-oil and pyrolysis gas | 100 | 0.1 | 600 |
| 4 | Biochar | 24.18 | 0.1 | 600 |
| 5 | Bio-oil and pyrolysis gas | 75.82 | 0.1 | 600 |
| 6 | Bio-oil | 23.07 | 0.1 | 600 |
| 7 | Pyrolysis gas | 52.75 | 1 | 600 |
| 8 | Fe/FeO | 90 | 1 | 900 |
| 9 | Air | 120 | 0.1 | 25 |
| 10 | Compressed air | 120 | 1.2 | 200 |
| 11 | Compressed air | 80 | 1.2 | 200 |
| 12 | Compressed air | 40 | 1.2 | 200 |
| 13 | Fe3O4 | 95 | 1 | 850 |
| 14 | Depleted air | 75 | 1.1 | 850 |
| 15 | Depleted air | 115 | 0.1 | 350 |
| 16 | Steam | 45 | 1 | 250 |
| 17 | Depleted air | 115 | 0.1 | 50 |
| 18 | Steam + H2 | 40 | 1 | 850 |
| 19 | Hot water + H2 | 40 | 0.1 | 400 |
| 20 | Cooled water + H2 | 40 | 0.1 | 60 |
| 21 | H2 | 3.35 | 0.1 | 25 |
| 22 | Recycled water | 36.65 | 0.1 | 25 |
| 23 | Make-up water | 8.35 | 0.1 | 25 |
| 24 | Total water | 45 | 1 | 25 |
| 25 | Hot waste gas | 62.75 | 1 | 850 |
| 26 | Cooled waste gas | 62.75 | 0.1 | 350 |
| 27 | Waste gas | 62.75 | 0.1 | 50 |
| 28 | Fe2O3 | 100 | 1 | 850 |
| Equipment | Value (kW) |
|---|---|
| Crusher | −25 |
| Pyrolysis reactor | −20 |
| Pump | −1 |
| Compressor-1 | −8 |
| Compressor-2 | −7 |
| Expander-1 | 15 |
| Expander-2 | 29 |
| Expander-3 | 20 |
| Net electricity | 3 |
| Item | Fuel Reactor | Steam Reactor | Air Reactor |
|---|---|---|---|
| kmol/h | |||
| H2 | 0.0415994 | 0.0149086 | 0 |
| CO | 0.284967 | 0 | 0 |
| CH4 | 2.29202 × 10−6 | 0 | 0 |
| CO2 | 1.15597 | 0 | 0 |
| Fe | 0 | 0 | 0 |
| FeO | 1.25241 | 1.22259 | 0 |
| Fe2O3 | 0 | 0.0149086 | 0.626205 |
| O2 | 2.29196 × 10−16 | 1.00022 × 10−17 | 0.276666 |
| N2 | 0 | 0 | 2.19061 |
| H2O | 0.212528 | 2.48297 | 0 |
| Proximate analysis (wad/%) | |
| Ash | 9.22 |
| Volatile Matter | 69.16 |
| Fixed Carbon | 14.86 |
| Moisture | 6.76 |
| Ultimate analysis (wdaf/%) | |
| C | 44.50 |
| O | 45.66 |
| H | 6.24 |
| N | 1.45 |
| S | 2.15 |
| Product | Simulation (wt% of Rice Straw) | Experiment (wt% of Rice Straw) | Relative Error (%) * | |
|---|---|---|---|---|
| Pyrolysis gas | CO | 17.01 | 18.68 | 8.94 |
| CO2 | 29.51 | 28.84 | 2.32 | |
| H2 | 1.53 | 1.73 | 11.56 | |
| CH4 | 3.66 | 3.83 | 4.44 | |
| Others | 1.04 | 0.92 | 13.04 | |
| Bio-oil | 23.07 | 21 | 9.86 | |
| Biochar | 24.18 | 25 | 3.28 | |
| Equipment | Aspen Model | Design Parameters |
|---|---|---|
| Crusher | Crusher | 25 °C, 0.1 MPa |
| Separator | SSplit | 600 °C, 0.1 MPa |
| Flash drum-1 | Flash | 600 °C, 0.1 MPa |
| Flash drum-2 | Flash | 25 °C, 0.1 MPa |
| Pyrolysis reactor | RYield, REquil and RGibbs | 600 °C, 0.1 MPa |
| Fuel reactor | RGibbs | 900 °C, 1 MPa |
| Steam reactor | RGibbs | 700 °C, 1 MPa |
| Air reactor | RGibbs | 980 °C, 1 MPa |
| Pump | Pump | 25 °C, 1 MPa |
| Heat recovery steam generator | MHeatX | |
| Compressor-1 | Compr | 1 MPa |
| Compressor-2 | Compr | 1.2 MPa |
| Expander-1, Expander-2 and Expander-3 | Compr | 0.1 MPa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Jin, H.; Li, N.; Ma, X.; Ma, Z.; Lu, P.; Yao, X.; Chen, S. New Process Combining Fe-Based Chemical Looping and Biomass Pyrolysis for Cogeneration of Hydrogen, Biochar, Bio-Oil and Electricity with In-Suit CO2 Separation. Molecules 2023, 28, 2793. https://doi.org/10.3390/molecules28062793
Zhou X, Jin H, Li N, Ma X, Ma Z, Lu P, Yao X, Chen S. New Process Combining Fe-Based Chemical Looping and Biomass Pyrolysis for Cogeneration of Hydrogen, Biochar, Bio-Oil and Electricity with In-Suit CO2 Separation. Molecules. 2023; 28(6):2793. https://doi.org/10.3390/molecules28062793
Chicago/Turabian StyleZhou, Xing, Huilong Jin, Na Li, Xiaolong Ma, Zichuan Ma, Pei Lu, Xiaomeng Yao, and Shenna Chen. 2023. "New Process Combining Fe-Based Chemical Looping and Biomass Pyrolysis for Cogeneration of Hydrogen, Biochar, Bio-Oil and Electricity with In-Suit CO2 Separation" Molecules 28, no. 6: 2793. https://doi.org/10.3390/molecules28062793