Lead-Free BNT–BT0.08/CoFe2O4 Core–Shell Nanostructures with Potential Multifunctional Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Characterization of the Core–Shell Materials
3. Results and Discussion
3.1. Structure and Microstructure Characterization
3.2. Complex Impedance Spectrum Analysis
3.3. Mössbauer Spectroscopy Results
3.4. Magnetic Characterization
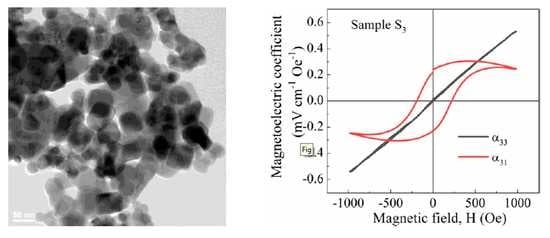
3.5. Magnetoelectric Voltage Response
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cotica, L.F. Surface spin disorder effects in magnetite and poly(thiophene)-coated magnetite nanoparticles. J. Appl. Phys. 2010, 108, 064325. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J. Magnetoelectric effect in composites of magnetostrictive and piezoelectric materials. J. Electroceram. 2002, 8, 107–119. [Google Scholar] [CrossRef]
- Wu, J.; Fan, Z.; Xiao, D.; Zhu, J.; Wang, J. Multiferroic bismuth ferrite-based materials for multifunctional applications: Ceramic bulks, thin films and nanostructures. Prog. Mater. Sci. 2016, 84, 335–402. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Grey, D.; Berry, D.; Gao, J.; Li, M.; Li, J.; Viehland, D. An Extremely Low Equivalent Magnetic Noise Magnetoelectric Sensor. Adv. Mater. 2011, 23, 4111–4114. [Google Scholar] [CrossRef]
- Marauska, S.; Jahns, R.; Kirschof, C.; Klaus, M.; Quandt, E.; Knochel, R.; Wagner, B. Highly sensitive wafer-level packaged MEMS magnetic field sensor based on magnetoelectric composites. Sens. Actuators A Phys. 2013, 189, 321–327. [Google Scholar] [CrossRef]
- Dong, S.; Zhai, J.; Li, J.F.; Viehland, D.; Priya, S. Multimodal system for harvesting magnetic and mechanical energy. Appl. Phys. Lett. 2008, 93, 103511–103513. [Google Scholar] [CrossRef] [Green Version]
- Liu, L. Multiferroic ferrite/perovskite oxide core/shell nanostructures. J. Mater. Chem. 2010, 20, 10665–10670. [Google Scholar] [CrossRef]
- Oliveira, P.N.; Silva, D.M.; Dias, G.S.; Santos, I.A.; Cotica, L.F. Synthesis and physical property measurements of CoFe2O4:BaTiO3 core–shell composite nanoparticles. Ferroelectrics 2016, 499, 76–82. [Google Scholar] [CrossRef]
- Cernea, M.; Ciuchi, I.V.; Vasile, B.S.; Surdu, V.A.; Bartha, C.; Iuga, A.; Galizia, P.; Galassi, C. Composite BNT-BT0.08/CoFe2O4 with core-shell nanostructure for piezoelectric and ferromagnetic applications. Mater. Sci. Eng. B 2019, 240, 7–15. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, G.; Chen, X.; Zhou, H. Observation of magnetoelectric coupling and the electrical properties in 0.65BaTiO3–0.35Bi0.5Na0.5TiO3/CoFe2O4 particulate composites. J. Mater. Sci. Mater. Electron. 2015, 26, 6107–6112. [Google Scholar] [CrossRef]
- Gao, X.; Rodriguez, B.J.; Liu, L.; Birajdar, B.; Pantel, D.; Ziese, M.; Alexe, M.; Hesse, D. Microstructure and properties of well ordered multiferroic Pb(Zr,Ti)O3/CoFe2O4 nanocomposites. ACS Nano 2010, 4, 1099–1107. [Google Scholar] [CrossRef]
- Ryu, H.; Murugavel, P.; Lee, J.H.; Chae, S.C.; Noh, T.W.; Oh, Y.S.; Kim, H.J.; Kim, K.H.; Jang, J.H.; Kim, M.; et al. Magnetoelectric effects of nanoparticulate Pb(Zr0.52Ti0.48)O3–NiFe2O4. Appl. Phys. Lett. 2006, 89, 102907. [Google Scholar] [CrossRef] [Green Version]
- Corral-Flores, V.; Bueno-Baques, D.; Ziolo, R.F. Synthesis and characterization of novel CoFe2O4–BaTiO3 multiferroic core-shell-type nanostructures. Acta Mater. 2010, 58, 764–769. [Google Scholar] [CrossRef]
- Shvartsman, V.V.; Alawneh, F.; Borisov, P.; Kozodaev, D.; Lupascu, D.C. Converse magnetoelectric effect in CoFe2O4–BaTiO3 composites with a core–shell structure. Smart Mater. Struct. 2011, 20, 075006. [Google Scholar] [CrossRef] [Green Version]
- Betal, S.; Dutta, M.; Cotica, L.F.; Bhalla, A.; Guo, R. BaTiO3 Coated CoFe2O4–Core-Shell Magnetoelectric Nanoparticles (CSMEN) Characterization. Integr. Ferroelectr. 2015, 166, 225–231. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Mandal, K. Large magnetoelectric properties in CoFe2O4:BaTiO3 core–shell nanocomposites. J. Magn. Magn. Mater. 2015, 377, 441–445. [Google Scholar] [CrossRef]
- Zhou, J.P.; Lv, L.; Liu, Q.; Zhang, Y.X.; Liu, P. Hydrothermal synthesis and properties of NiFe2O4@BaTiO3 composites with well–matched interface. Sci. Technol. Adv. Mater. 2012, 13, 045001. [Google Scholar] [CrossRef] [PubMed]
- Jnaneshwara, D.M.; Avadhani, D.N.; Prasad, B.D.; Nagabhushana, B.M.; Nagabhushana, H.; Sharma, S.C.; Shivakumara, C.; Rao, J.L.; Gopal, N.O.; Ke, S.C.; et al. Electron paramagnetic resonance, magnetic and electrical properties of CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 2013, 339, 40–45. [Google Scholar] [CrossRef]
- Cernea, M.; Montanari, G.; Galassi, C.; Costa, A.L. Synthesis of La and Nb doped PZT powder by the gel–combustion method. Nanotechnology 2006, 17, 1731–1735. [Google Scholar] [CrossRef]
- Chakrabarti, N.; Maiti, H.S. Improved process for preparation of lead zirconate titanate powder, IN189960-B. 24 May 2003. [Google Scholar]
- Brand, R.A. Improving the validity of hyperfine field distributions from magnetic alloys. Nucl. Instrum. Method B 1987, 28, 398–416. [Google Scholar] [CrossRef]
- Jones, G.O.; Thomas, P.A. Investigation of the structure and phase transitions in the novel A-site substituted distorted perovskite compound Na0.5Bi0.5TiO3. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 168–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teillet, J.; Bouree, F.; Krishnan, R. Magnetic structure of CoFe2O4. J. Magn. Magn. Mater. 1993, 123, 93–96. [Google Scholar] [CrossRef]
- Teodorescu, C.M.; Esteva, J.M.; Karnatak, R.C.; El Afif, A. An approximation of the Voigt I profile for the fitting of experimental X-ray absorption data. Nucl. Instrum. Method Phys. Res. Sect. A 1994, 345, 141–147. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the valence bands of gold. Phys. Rev. 1972, 55, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Mardare, D.; Luca, D.; Teodorescu, C.M.; Macovei, D. On the hydrophilicity of nitrogen-doped TiO2 thin films. Surf. Sci. 2007, 601, 4515–4520. [Google Scholar] [CrossRef]
- Wang, W.P.; Yang, H.; Xian, T.; Jiang, J.L. XPS and magnetic properties of CoFe2O4 nanoparticles synthesized by a polyacrylamide gel route. Mater. Trans. 2012, 53, 1586–1589. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.F.; Hu, X.L.; Wen, Y.W.; Shan, B.; Hu, P. Electrospun sillenite Bi12MO20 (M = Ti, Ge, Si) nanofibers: General synthesis, band structure, and photocatalytic activity. Phys. Chem. Chem. Phys. 2013, 15, 20698–20705. [Google Scholar] [CrossRef]
- Wagner, C.D.; Davis, L.E.; Zeller, M.V.; Taylor, J.A.; Raymond, R.M.; Gale, L.H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis. Surf. Interface Anal. 1981, 3, 211–225. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, B.P.; Choudhary, R.N.P.; Thakur, A.K. Characterization of electrical properties of Pb-modified BaSnO3 using impedance spectroscopy. Mater. Chem. Phys. 2006, 99, 150–159. [Google Scholar] [CrossRef]
- Behera, B.; Nayak, P.; Choudhary, R.N.P. Impedance spectroscopy study of NaBa2V5O15 ceramic. J. Alloys Compds. 2007, 436, 226–232. [Google Scholar] [CrossRef]
- Hao, J.; Xu, Z.; Chu, R.; Li, W.; Du, J. Lead-free electrostrictive (Bi0.5Na0.5)TiO3–(Bi0.5K0.5)TiO3–(K0.5Na0.5)NbO3 ceramics with good thermostability and fatigue-free behavior. J. Mater. Sci. 2015, 50, 5328–5336. [Google Scholar] [CrossRef]
- Jamnik, J.; Maier, J. Generalised equivalent circuits for mass and charge transport: Chemical capacitance and its implications. Phys. Chem. Chem. Phys 2001, 3, 1668–1678. [Google Scholar] [CrossRef]
- Padmasree, K.P.; Kanchan, D.K.; Kulkami, A.R. Impedance and Modulus studies of the solid electrolyte system 20CdI2–80[xAg2O–y(0.7V2O5–0.3B2O3)], where 1 ≤ x/y ≤ 3. Solid State Ionics 2006, 177, 475–482. [Google Scholar] [CrossRef]
- Ncib, W.; Ben Jazia Kharrat, A.; Wederni, M.A.; Chniba-Boudjada, N.; Khirouni, K.; Boujelben, W. Investigation of structural, electrical and dielectric properties of sol-gel prepared La0.67-xEuxBa0.33Mn0.85Fe0.15O3 (x = 0.0, 0.1) manganites. J. Alloys Compd. 2018, 768, 249–262. [Google Scholar] [CrossRef]
- Costa, M.M.; Pires, G.F.M.; Terezo, A.J., Jr.; Grac¸, M.P.F.; Sombra, A.S.B. Impedance and modulus studies of magnetic ceramic oxide Ba2Co2Fe12O22(Co2Y) doped with Bi2O3. J. Appl. Phys. 2011, 110, 034107. [Google Scholar] [CrossRef]
- Yordanov, S.P.; Ivanov, I.; Carapanov, C.P. Dielectric properties of the ferroelectric Bi2Ti2O7 ceramics. J. Phys. D 1998, 31, 800–806. [Google Scholar] [CrossRef]
- Shi, M.; Zuo, R.; Xu, Y.; Jiang, Y.; Yu, G.; Su, H.; Zhong, J. Preparation and characterization of CoFe2O4 powders and films via the sol–gel method. J. Alloys Compd. 2012, 512, 165–170. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Gibb, T.C. Mossbauer Spectroscopy; Chapman and Hall Ltd.: London, UK, 1971. [Google Scholar]
- Sawatzky, G.A.; Van Der Woude, F.; Morrish, A. H Recoilless-Fraction Ratios for Fe57 in Octahedral and Tetrahedral Sites of a Spinel and a Garnet. Phys. Rev. 1969, 183, 383–386. [Google Scholar] [CrossRef]
- Cernea, M.; Vasile, B.S.; Ciuchi, I.V.; Surdu, V.A.; Bartha, C.; Iuga, A.; Galizia, P.; Galassi, C. Synthesis and characterization of novel ferrite–piezoelectric multiferroic core–shell-type structure. J. Mater. Sci. 2018, 53, 9650–9661. [Google Scholar] [CrossRef]
- Chagas, E.F.; Ponce, A.S.; Prado, R.J.; Silva, G.M.; Bettini, J. Baggio-Saitovitch, Thermal effect on magnetic parameters of high-coercivity cobalt ferrite. J. Appl. Phys. 2014, 116, 033901. [Google Scholar] [CrossRef] [Green Version]
- Stoner, E.C.; Wohlfarth, E.P. A mechanism of magnetic hysteresis in heterogeneous alloys. Philos. Trans. R. Soc. A 1948, 240, 599–644. [Google Scholar] [CrossRef]
- Amorín, H.; Algueró, M.; Del Campo, R.; Vila, E.; Ramos, P.; Dolle, M.; Romaguera-Barcelay, Y.; Pérez de la Cruz, J.; Castro, A. High-sensitivity piezoelectric perovskite for magnetoelectric composites. Sci. Technol. Adv. Mater. 2015, 16, 016001. [Google Scholar] [CrossRef] [PubMed]
- Bichurin, M.I.; Petrov, V.M.; Priya, S. Magnetoelectric Multiferroic Composites. In Ferroelectrics—Physical Effects; Lallart, M., Ed.; InTech: Chicago, IL, USA, 2011; pp. 277–302. [Google Scholar]
- Santa-Rosa, W.; da Silva, P.S.; M’Peko, J.C.; Amorín, H.; Algueró, M.; Venet, M. Enhanced piezomagnetic coefficient of cobalt ferrite ceramics by Ga and Mn doping for magnetoelectric applications. J. App. Phys. 2019, 125, 075107. [Google Scholar] [CrossRef]

















| Powders | S1: 1:0.5 | S2: 1:1 | S3: 1:1.5 | |||
|---|---|---|---|---|---|---|
| BNT–BT0.08 | CoFe2O4 | BNT–BT0.08 | CoFe2O4 | BNT–BT0.08 | CoFe2O4 | |
| a [Å] | 5.5192 | 8.3774 | 5.5231 | 8.3872 | 5.5260 | 8.3777 |
| b [Å] | 5.5192 | 8.3774 | 5.5231 | 8.3872 | 5.5260 | 8.3777 |
| c [Å] | 3.9126 | 8.3774 | 3.9137 | 8.3872 | 3.9064 | 8.3777 |
| c/a | 0.7089 | 0.7086 | 0.7069 | |||
| Volume [Å3] | 119.1825 | 587.9251 | 119.3868 | 588.3788 | 119.2883 | 587.9922 |
| Rexp | 5.8666 | 4.6676 | 4.2794 | |||
| Rp | 3.3093 | 2.6739 | 2.5936 | |||
| Rwp | 4.2908 | 3.4512 | 3.3441 | |||
| χ2 | 0.5349 | 0.5467 | 0.6107 | |||
| Average crystallite size, D [nm] | 28.32 | 26.76 | 31.64 | 25.26 | 29.04 | 22.16 |
| Sample | Permittivity εr, at 1 kHz | Tan δ, at 1kHz | Conductivity σdc, (S/cm) |
|---|---|---|---|
| S1, ceramic | 515 | 0.632 | 1.66 × 10−9 |
| S2, ceramic | 468 | 1.086 | 6.27 × 10−9 |
| S3, ceramic | 318 | 1.750 | 1.36 × 10−8 |
| Sample Code | Component | IS (mm/s) | QS (mm/s) | Bhf (T) | RA (%) | γ | Formula Unit |
|---|---|---|---|---|---|---|---|
| S1 | T | 0.33(1) | −0.01(2) | 50.1(1) | 34(2) | 0.708 | (Co0.292Fe0.708)[Co0.708Fe1.292]O4 |
| O | 0.271(4) | −0.014(8) | 47.77(4) | 66(2) | |||
| S2 | T | 0.331(5) | −0.006(9) | 50.1(4) | 38(1) | 0.789 | (Co0.211Fe0.789)[Co0.789Fe1.211]O4 |
| O | 0.271(2) | 0.0 | 47.80(2) | 62(1) | |||
| S3 | T | 0.341(6) | 0.0 | 50.48(5) | 29(1) | 0.606 | (Co0.394Fe0.606)[Co0.606Fe1.394]O4 |
| O | 0.275(3) | 0.007(5) | 47.78(2) | 71(1) |
| Sample | CoFe2O4 Mass (out of 1) | MS (emu/g) | MR (emu/g) | HC (Oe) |
|---|---|---|---|---|
| S1, powder | 0.35 | 24.7 | 10.5 | 1016 |
| S2, powder | 0.52 | 35.8 | 14.7 | 1199 |
| S3, powder | 0.62 | 47.3 | 20 | 1083 |
| CoFe2O4 powder [32] | 1 | 82.8 | 52 | 814 |
| CoFe2O4 core – BaTiO3 shell powder composites [8] | 0.6 | 33.9 | 5.98 | 399 |
| CoFe2O4 core – BNT–BT0.08 shell composites [41] | 0.975 | 73 | 61 | 60 |
| CoFe2O4 – BNT–BT0.65 particulate composites [10] | 0.1 | 2.5 | 0.5 | 3972 |
| 0.2 | 7.5 | 1.5 | 4500 | |
| 0.3 | 13 | 2.5 | 4676 | |
| 0.4 | 17 | 3 | 6000 | |
| S1, ceramic | 0.35 | 21.1 | 3.36 | 209 |
| S2, ceramic | 0.52 | 34.9 | 5.92 | 223 |
| S3, ceramic | 0.62 | 47.2 | 7.15 | 187 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cernea, M.; Radu, R.; Amorín, H.; Greculeasa, S.G.; Vasile, B.S.; Surdu, V.A.; Ganea, P.; Trusca, R.; Hattab, M.; Galassi, C. Lead-Free BNT–BT0.08/CoFe2O4 Core–Shell Nanostructures with Potential Multifunctional Applications. Nanomaterials 2020, 10, 672. https://doi.org/10.3390/nano10040672
Cernea M, Radu R, Amorín H, Greculeasa SG, Vasile BS, Surdu VA, Ganea P, Trusca R, Hattab M, Galassi C. Lead-Free BNT–BT0.08/CoFe2O4 Core–Shell Nanostructures with Potential Multifunctional Applications. Nanomaterials. 2020; 10(4):672. https://doi.org/10.3390/nano10040672
Chicago/Turabian StyleCernea, Marin, Roxana Radu, Harvey Amorín, Simona Gabriela Greculeasa, Bogdan Stefan Vasile, Vasile Adrian Surdu, Paul Ganea, Roxana Trusca, Marwa Hattab, and Carmen Galassi. 2020. "Lead-Free BNT–BT0.08/CoFe2O4 Core–Shell Nanostructures with Potential Multifunctional Applications" Nanomaterials 10, no. 4: 672. https://doi.org/10.3390/nano10040672