Hydrothermal Synthesis of Aqueous-Soluble Copper Indium Sulfide Nanocrystals and Their Use in Quantum Dot Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CIS NCs Synthesis
2.3. Characterization
2.4. NCs Deposition
2.5. Solar Cells
3. Results and Discussion
3.1. Influence of the Synthesis Parameters on the Properties of Aqueous NCs
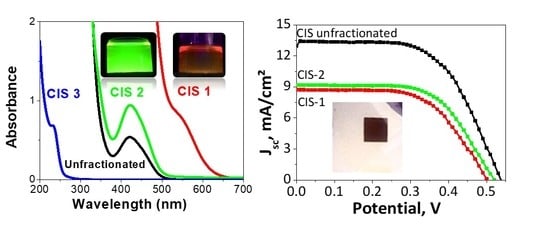
3.2. Fractionation and Characterization of NCs
3.3. Solar Cell Integration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aldakov, D.; Lefrançois, A.; Reiss, P. Ternary and quaternary metal chalcogenide nanocrystals: Synthesis, properties and applications. J. Mater. Chem. C 2013, 1, 3756–3776. [Google Scholar] [CrossRef]
- Sandroni, M.; Wegner, K.D.; Aldakov, D.; Reiss, P. Prospects of chalcopyrite-type nanocrystals for energy applications. ACS Energy Lett. 2017, 2, 1076–1088. [Google Scholar] [CrossRef]
- Coughlan, C.; Ibáñez, M.; Dobrozhan, O.; Singh, A.; Cabot, A.; Ryan, K.M. Compound copper chalcogenide nanocrystals. Chem. Rev. 2017, 117, 5865–6109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fang, W.; Wang, W.; Qian, N.; Ji, X. Highly Efficient Zn-Cu-In-Se Quantum Dot-Sensitized Solar Cells through Surface Capping with Ascorbic Acid. ACS Appl. Mater. Interfaces 2019, 11, 6927–6936. [Google Scholar] [CrossRef]
- Reiss, P.; Carrière, M.; Lincheneau, C.; Vaure, L.; Tamang, S. Synthesis of semiconductor nanocrystals, focusing on nontoxic and earth-abundant materials. Chem. Rev. 2016, 116, 10731–10819. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Xin, W.; Yao, D.; Liu, Y.; Zhang, L.; Liu, W.; Zhang, W.; Zheng, W.; Yang, B.; et al. Facile synthesis of Cu-In-S/ZnS core/shell quantum dots in 1-dodecanethiol for efficient light-emitting diodes with an external quantum efficiency of 7.8%. Chem. Mater. 2018, 30, 8939–8947. [Google Scholar] [CrossRef]
- Chuang, P.; Lin, C.C.; Liu, R. Emission-tunable CuInS2/ZnS quantum dots: Structure, optical properties, and application in white light-emitting diodes with high color rendering index. ACS Appl. Mater. Interfaces 2014, 6, 1764–1769. [Google Scholar] [CrossRef]
- Gromova, M.; Lefrancois, A.; Vaure, L.; Agnese, F.; Aldakov, D.; Maurice, A.; Djurado, D.; Lebrun, C.; de Geyer, A.; Schülli, T.U.; et al. Growth mechanism and surface state of CuInS2 nanocrystals synthesized with dodecanethiol. J. Am. Chem. Soc. 2017, 139, 15748–15759. [Google Scholar] [CrossRef]
- Moodelly, D.; Kowalik, P.; Bujak, P.; Pron, A.; Reiss, P. Synthesis, photophysical properties and surface chemistry of chalcopyrite-type semiconductor nanocrystals. J. Mater. Chem. C 2019, 7, 11665–11709. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Q.; Huang, X.; Li, D.; Luo, Y.; Meng, Q. Aqueous colloidal CuInS2 for quantum dot sensitized solar cells. J. Mater. Chem. 2011, 21, 15903. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Cao, C.; Shi, C. Synthesis of band-gap tunable Cu–In–S ternary nanocrystals in aqueous solution. RSC Adv. 2012, 2, 2666. [Google Scholar] [CrossRef]
- Zhou, W.-H.; Jiao, J.; Zhao, Y.; Cheng, X.-Y.; Kou, D.-X.; Zhou, Z.-J.; Wu, S.-X. Synthesis of metastable wurtzite CuInS2 nanocrystals and films from aqueous solution. RSC Adv. 2014, 4, 7617. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Huang, L.; Pan, D. Green and facile synthesis of water-soluble Cu-In-S/ZnS core/shell quantum dots. Inorg. Chem. 2013, 52, 7819–7821. [Google Scholar] [CrossRef] [PubMed]
- Raevskaya, A.; Rosovik, O.; Kozytskiy, A.; Stroyuk, O.; Dzhagan, V.; Zahn, D.R.T.T. Non-stoichiometric Cu–In–S@ZnS nanoparticles produced in aqueous solutions as light harvesters for liquid-junction photoelectrochemical solar cells. RSC Adv. 2016, 6, 100145–100157. [Google Scholar] [CrossRef] [Green Version]
- Mange, Y.J.; Dewi, M.R.; Macdonald, T.J.; Skinner, W.M.; Nann, T. Rapid microwave assisted synthesis of nearly monodisperse aqueous CuInS2/ZnS nanocrystals. CrystEngComm 2015, 17, 7820–7823. [Google Scholar] [CrossRef]
- Xiong, W.-W.; Yang, G.-H.; Wu, X.-C.; Zhu, J.-J. Aqueous synthesis of color-tunable CuInS2/ZnS nanocrystals for the detection of human interleukin 6. ACS Appl. Mater. Interfaces 2013, 5, 8210–8216. [Google Scholar] [CrossRef]
- Chang, J.Y.; Li, C.H.; Chiang, Y.H.; Chen, C.H.; Li, P.N. Toward the facile and ecofriendly fabrication of quantum dot-sensitized solar cells via thiol coadsorbent assistance. ACS Appl. Mater. Interfaces 2016, 8, 18878–18890. [Google Scholar] [CrossRef]
- Sandroni, M.; Gueret, R.; Wegner, K.D.; Reiss, P.; Fortage, J.; Aldakov, D.; Collomb, M.-N. Cadmium-free CuInS2/ZnS quantum dots as efficient and robust photosensitizers in combination with a molecular catalyst for visible light-driven H2 production in water. Energy Environ. Sci. 2018, 11, 1752–1761. [Google Scholar] [CrossRef]
- Higashimoto, S.; Murano, M.; Arase, T.; Mukai, S.; Azuma, M.; Takahashi, M. Highly qualified copper-indium sulfide colloids prepared in water under microwave irradiation and their applications to the TiO2 based quantum dot-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2017, 169, 203–209. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Qiao, Y.; Su, X. One-pot synthesis of ternary CuInS2 quantum dots with near-infrared fluorescence in aqueous solution. RSC Adv. 2012, 2, 819. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Huang, L.; Pan, D. Low-cost and gram-scale synthesis of water-soluble Cu-In-S/ZnS core/shell quantum dots in an electric pressure cooker. Nanoscale 2014, 6, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, Z.; Lin, Z.; Su, X. CuInS2 quantum dots/poly((L)-glutamic acid)-drug conjugates for drug delivery and cell imaging. Analyst 2014, 139, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Song, J.; Wang, H.; Ye, X.; Wang, H.; Zhang, W.; Yang, M.; Xia, R.; Zhu, L.; Xu, X. Aqueous synthesis of color tunable Cu doped Zn–In–S/ZnS nanoparticles in the whole visible region for cellular imaging. J. Mater. Chem. B 2015, 3, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Spangler, L.C.; Chu, R.; Lu, L.; Kiely, C.J.; Berger, B.W.; McIntosh, S. Enzymatic biomineralization of biocompatible CuInS2, (CuInZn)S2 and CuInS2/ZnS core/shell nanocrystals for bioimaging. Nanoscale 2017, 9, 9340–9351. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Xia, T.; Su, X.; Ma, Q. Aqueous synthesis of high quality multicolor Cu-Zn-In-S quantum dots. J. Lumin. 2017, 188, 162–167. [Google Scholar] [CrossRef]
- Girma, W.M.; Fahmi, M.Z.; Permadi, A.; Abate, M.A.; Chang, J.-Y. Synthetic strategies and biomedical applications of I–III–VI ternary quantum dots. J. Mater. Chem. B 2017, 5, 6193–6216. [Google Scholar] [CrossRef]
- Macdonald, T.J.; Mange, Y.J.; Dewi, M.R.; Islam, H.U.; Parkin, I.P.; Skinner, W.M.; Nann, T. CuInS2/ZnS nanocrystals as sensitisers for NiO photocathodes. J. Mater. Chem. A 2015, 3, 13324–13331. [Google Scholar] [CrossRef]
- Luo, J.; Wei, H.; Huang, Q.; Hu, X.; Zhao, H.; Yu, R.; Li, D.; Luo, Y.; Meng, Q. Highly efficient core-shell CuInS2-Mn doped CdS quantum dot sensitized solar cells. Chem. Commun. (Camb.) 2013, 49, 3881–3883. [Google Scholar] [CrossRef] [Green Version]
- Higashimoto, S.; Nakase, T.; Mukai, S.; Takahashi, M. Copper-indium-sulfide colloids on quantum dot sensitized TiO2 solar cell: Effects of capping with mercapto-acid linker molecules. J. Colloid Interface Sci. 2019, 535, 176–181. [Google Scholar] [CrossRef]
- Nam, D.-E.; Song, W.-S.; Yang, H. Facile, air-insensitive solvothermal synthesis of emission-tunable CuInS2/ZnS quantum dots with high quantum yields. J. Mater. Chem. 2011, 21, 18220. [Google Scholar] [CrossRef]
- Akdas, T.; Walter, J.; Segets, D.; Distaso, M.; Winter, B.; Birajdar, B.; Spiecker, E.; Peukert, W. Investigation of the size–property relationship in CuInS2 quantum dots. Nanoscale 2015, 7, 18105–18118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, S.L.; Bailey, S.G.; Raffaelle, R.P.; Banger, K.K.; Hepp, A.F. Synthesis and characterization of colloidal CuInS2 nanoparticles from a molecular single-source precursor. J. Phys. Chem. B 2004, 108, 12429–12435. [Google Scholar] [CrossRef]
- Xie, R.; Rutherford, M.; Peng, X. Formation of high-quality I-III-VI semiconductor nanocrystals by tuning relative reactivity of cationic precursors. J. Am. Chem. Soc. 2009, 131, 5691–5697. [Google Scholar] [CrossRef] [PubMed]
- Raevskaya, A.; Lesnyak, V.; Haubold, D.; Dzhagan, V.; Stroyuk, O.; Gaponik, N.; Zahn, D.R.T.; Eychmüller, A. A fine size selection of brightly luminescent water-soluble Ag-In-S and Ag-In-S/ZnS quantum dots. J. Phys. Chem. C 2017, 121, 9032–9042. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.S.; Choi, S.H.; Bang, J.H. New insight into copper sulfide electrocatalysts for quantum dot-sensitized solar cells: Composition-dependent electrocatalytic activity and stability. ACS Appl. Mater. Interfaces 2014, 6, 22078–22087. [Google Scholar] [CrossRef] [PubMed]
- Kolny-Olesiak, J.; Weller, H. Synthesis and application of colloidal CuInS2 semiconductor nanocrystals. ACS Appl. Mater. Interfaces 2013, 5, 12221–12237. [Google Scholar] [CrossRef]
- Jara, D.H.; Stamplecoskie, K.G.; Kamat, P.V. Two distinct transitions in CuxInS2 quantum dots. Bandgap versus sub-bandgap excitations in copper-deficient structures. J. Phys. Chem. Lett. 2016, 7, 1452–1459. [Google Scholar] [CrossRef]
- Fuhr, A.; Yun, H.J.; Makarov, N.S.; Li, H.; McDaniel, H.; Klimov, V.I. Light-emission mechanism in CuInS2 quantum dots evaluated by spectral electrochemistry. ACS Photonics 2017, 4, 2425–2435. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Yang, C.; Hu, S.; Gao, Y.; Zhang, Y.; Wang, Y.; Demir, H.V.; Liu, L.; Yong, K.-T. The composition effect on the optical properties of aqueous synthesized Cu–In–S and Zn–Cu–In–S quantum dot nanocrystals. Phys. Chem. Chem. Phys. 2015, 17, 25133–25141. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wei, S.; Zunger, A.; Katayama-Yoshida, H. Defect physics of the CuInSe2 chalcopyrite semiconductor. Phys. Rev. B 1998, 57, 9642–9656. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Ahn, S.-H.; Chung, K.; Cho, Y.-S.; Choi, C.-J. The photoluminescence of CuInS2 nanocrystals: Effect of non-stoichiometry and surface modification. J. Mater. Chem. 2012, 22, 1516. [Google Scholar] [CrossRef]
- de Trizio, L.; Prato, M.; Genovese, A.; Casu, A.; Povia, M.; Simonutti, R.; Alcocer, M.J.P.; Andrea, C.D.; Tassone, F.; Manna, L. Strongly fluorescent quaternary Cu−In−Zn−S nanocrystals prepared from Cu1−xInS2 nanocrystals by partial cation exchange. Chem. Mater. 2012, 24, 2400–2406. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, A.; Wang, M.; Yang, C.; Teng, F. Heating-up synthesis of cadimum-free and color-tunable quaternary and five-component Cu–In–Zn–S-based semiconductor nanocrystals. J. Mater. Chem. C 2015, 3, 10114–10120. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, H.; Zhang, W.; Tan, Z.; Li, Y.; Yu, C.; Zhai, T.; Bando, Y.; Yang, S.; Zou, B. Highly emissive and color-tunable CuInS2-based colloidal semiconductor nanocrystals: Off-stoichiometry effects and improved electroluminescence performance. Adv. Funct. Mater. 2012, 22, 2081–2088. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, Z.; Wang, W.; Du, J.; Ren, Z.; Shen, Q.; Zhong, X. Copper deficient Zn-Cu-In-Se quantum dot sensitized solar cells for high efficiency. J. Mater. Chem. A 2017, 5, 21442–21451. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Sun, Y.; Geng, X.; Hu, Y.; Meng, H.; Ge, J.; Qu, L. Synthesis of luminescent carbon dots with ultrahigh quantum yield and inherent folate receptor-positive cancer cell targetability. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Knowles, K.E.; Hartstein, K.H.; Kilburn, T.B.; Marchioro, A.; Nelson, H.D.; Whitham, P.J.; Gamelin, D.R. Luminescent colloidal semiconductor nanocrystals containing copper: Synthesis, photophysics, and applications. Chem. Rev. 2016, 116, 10820–10851. [Google Scholar] [CrossRef]
- Tran, T.K.C.; Le, Q.P.; Nguyen, Q.L.; Li, L.; Reiss, P. Time-resolved photoluminescence study of CuInS2/ZnS nanocrystals. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 025007. [Google Scholar] [CrossRef] [Green Version]
- Kramer, I.J.; Sargent, E.H. The architecture of colloidal quantum dot solar cells: Materials to devices. Chem. Rev. 2014, 114, 863–882. [Google Scholar] [CrossRef]
- Santra, P.K.; Kamat, P.V. Tandem-layered quantum dot solar cells: Tuning the photovoltaic response with luminescent ternary cadmium chalcogenides. J. Am. Chem. Soc. 2013, 135, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gonzalez-Pedro, V.; Kubo, T.; Fabregat-Santiago, F.; Bisquert, J.; Sanehira, Y.; Nakazaki, J.; Segawa, H. Enhanced carrier transport distance in colloidal PbS quantum dot-based solar cells using ZnO nanowires. J. Phys. Chem. C 2015, 119, 27265–27274. [Google Scholar] [CrossRef]
- Aldakov, D.; Sajjad, M.T.; Ivanova, V.; Bansal, A.K.; Park, J.; Reiss, P.; Samuel, I.D.W. Mercaptophosphonic acids as efficient linkers in quantum dot sensitized solar cells. J. Mater. Chem. A 2015, 3, 19050–19060. [Google Scholar] [CrossRef] [Green Version]





| Fraction | Cu | In | S |
|---|---|---|---|
| CIS-1 | 1.00 | 5.06 ± 0.79 | 8.24 ± 1.42 |
| CIS-2 | 1.00 | 7.40 ± 2.86 | 38.05 ± 12.29 |
| CIS-3 | 1.00 | 0.02 ± 0.03 | 56.70 ± 5.61 |
| Sample | Cell | VOC (V) | JSC (mA/cm2) | FF (%) | η (%) |
|---|---|---|---|---|---|
| CIS 1 | champion cell | 0.50 | 8.73 | 59 | 2.60 |
| average | 0.51 ± 0.01 | 8.53 ± 0.30 | 58 ± 1 | 2.52 ± 0.11 | |
| CIS 2 | champion cell | 0.52 | 9.18 | 61 | 2.91 |
| average | 0.52 ± 0.01 | 9.04 ± 0.12 | 60 ± 1 | 2.85 ± 0.07 | |
| CIS-1 + CIS-2 | champion cell | 0.53 | 9.09 | 67 | 3.19 |
| average | 0.52 ± 0.01 | 8.68 ± 0.58 | 65 ± 4 | 2.91 ± 0.40 | |
| Unfractionated CIS | champion cell | 0.52 | 13.96 | 64 | 4.67 |
| average | 0.53 ± 0.11 | 13.62 ± 0.32 | 63 ± 1 | 4.53 ± 0.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, C.I.L.; S. Machado, W.; Wegner, K.D.; Gontijo, L.A.P.; Bettini, J.; Schiavon, M.A.; Reiss, P.; Aldakov, D. Hydrothermal Synthesis of Aqueous-Soluble Copper Indium Sulfide Nanocrystals and Their Use in Quantum Dot Sensitized Solar Cells. Nanomaterials 2020, 10, 1252. https://doi.org/10.3390/nano10071252
Santos CIL, S. Machado W, Wegner KD, Gontijo LAP, Bettini J, Schiavon MA, Reiss P, Aldakov D. Hydrothermal Synthesis of Aqueous-Soluble Copper Indium Sulfide Nanocrystals and Their Use in Quantum Dot Sensitized Solar Cells. Nanomaterials. 2020; 10(7):1252. https://doi.org/10.3390/nano10071252
Chicago/Turabian StyleSantos, Calink I. L., Wagner S. Machado, Karl David Wegner, Leiriana A. P. Gontijo, Jefferson Bettini, Marco A. Schiavon, Peter Reiss, and Dmitry Aldakov. 2020. "Hydrothermal Synthesis of Aqueous-Soluble Copper Indium Sulfide Nanocrystals and Their Use in Quantum Dot Sensitized Solar Cells" Nanomaterials 10, no. 7: 1252. https://doi.org/10.3390/nano10071252