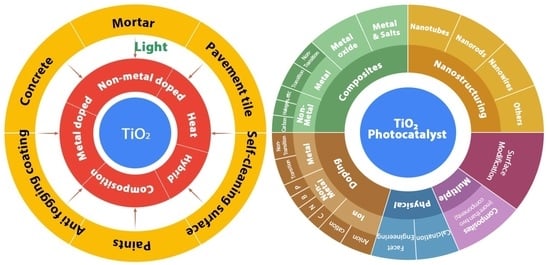
Recent Progress in the Abatement of Hazardous Pollutants Using Photocatalytic TiO2-Based Building Materials
Abstract
:1. Introduction
2. Building Materials
3. Integration of Photocatalyst on Building Materials
4. TiO2 Photoactivity under Solar Light
5. NOx Removal
6. Volatile Organic Compounds
TiO2 Based Photocatalytic Composites for VOC Removal
7. Self-Cleaning
8. Issues and Limitations
9. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Nathanael, A.J.; Kannaiyan, K.; Kunhiraman, A.K.; Kumaravel, V. Nanomaterials for detection and removal of gases. In Nanomaterials for Sustainable Energy and Environmental Remediation; Naushad, M., Saravanan, R., Raju, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 219–260. [Google Scholar] [CrossRef]
- Gallego, E.; Roca, X.; Perales, J.F.; Guardino, X. Determining indoor air quality and identifying the origin of odour episodes in indoor environments. J. Environ. Sci. 2009, 21, 333–339. [Google Scholar] [CrossRef]
- Tran, V.V.; Park, D.; Lee, Y.-C. Indoor Air Pollution, Related Human Diseases, and Recent Trends in the Control and Improvement of Indoor Air Quality. Int. J. Environ. Res. Public Health 2020, 17, 2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, D.Y.C. Outdoor-indoor air pollution in urban environment: Challenges and opportunity. Front. Environ. Sci. 2015, 2. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Borthakur, A. A review on biodegradation and photocatalytic degradation of organic pollutants: A bibliometric and comparative analysis. J. Clean. Prod. 2018, 196, 1669–1680. [Google Scholar] [CrossRef]
- Lee, J.-S.; Yoon, N.-R.; Kang, B.-H.; Lee, S.-W.; Gopalan, S.-A.; Kim, S.-W.; Lee, S.-H.; Kwon, D.-H.; Kang, S.-W. Au-Polypyrrole Framework Nanostructures for Improved Localized Surface Plasmon Resonance Volatile Organic Compounds Gas Sensing. J. Nanosci. Nanotechnol. 2015, 15, 7738–7742. [Google Scholar] [CrossRef]
- Hallquist, M.; Munthe, J.; Hu, M.; Wang, T.; Chan, C.K.; Gao, J.; Boman, J.; Guo, S.; Hallquist, Å.M.; Mellqvist, J.; et al. Photochemical smog in China: Scientific challenges and implications for air-quality policies. Natl. Sci. Rev. 2016, 3, 401–403. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Sun, D.Z.; Chi, G.Q. Formaldehyde degradation by UV/TiO2/O3 process using continuous flow mode. J. Environ. Sci. 2007, 19, 1136–1140. [Google Scholar] [CrossRef]
- Joseph, S.; Benzigar, M.R.; Ilbeygi, H.; Gopalan, S.A.; Lakhi, K.S.; Ramadass, K.; Talapaneni, S.N.; Vinu, A. Mesoporous Carbons with Hexagonally Ordered Pores Prepared from Carbonated Soft-Drink for CO2 Capture at High Pressure. J. Nanosci. Nanotechnol. 2018, 18, 7830–7837. [Google Scholar] [CrossRef]
- Joseph, S.; Saianand, G.; Benzigar, M.R.; Ramadass, K.; Singh, G.; Gopalan, A.-I.; Yang, J.H.; Mori, T.; Al-Muhtaseb, A.a.H.; Yi, J.; et al. Recent Advances in Functionalized Nanoporous Carbons Derived from Waste Resources and Their Applications in Energy and Environment. Adv. Sustain. Syst. 2020, 2000169. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Lee, H.-G.; Sai-Anand, G.; Komathi, S.; Gopalan, A.-I.; Kang, S.-W.; Lee, K.-P. Efficient visible-light-driven photocatalytic degradation of nitrophenol by using graphene-encapsulated TiO2 nanowires. J. Hazard. Mater. 2015, 283, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Saianand, G.; Sonar, P.; Wilson, G.J.; Gopalan, A.-I.; Roy, V.A.L.; Unni, G.E.; Mamun Reza, K.; Bahrami, B.; Venkatramanan, K.; Qiao, Q. Current advancements on charge selective contact interfacial layers and electrodes in flexible hybrid perovskite photovoltaics. J. Energy Chem. 2021, 54, 151–173. [Google Scholar] [CrossRef]
- Lee, H.-G.; Gopalan, A.-I.; Sai-Anand, G.; Lee, B.-C.; Kang, S.-W.; Lee, K.-P. Facile synthesis of functionalized graphene-palladium nanoparticle incorporated multicomponent TiO2 composite nanofibers. Mater. Chem. Phys. 2015, 154, 125–136. [Google Scholar] [CrossRef]
- Lang, X.; Saianand, G.; Fu, W.; Ramakrishna, S. Photocatalytic water splitting utilizing electrospun semiconductors for solar hydrogen generation: Fabrication, Modification and Performance. Bull. Chem. Soc. Jpn. 2020. [Google Scholar] [CrossRef]
- Xu, B.; Sai-Anand, G.; Unni, G.E.; Jeong, H.-M.; Kim, J.-S.; Kim, S.-W.; Kwon, J.-B.; Bae, J.-H.; Kang, S.-W. Pyridine-based additive optimized P3HT:PC61BM nanomorphology for improved performance and stability in polymer solar cells. Appl. Surf. Sci. 2019, 484, 825–834. [Google Scholar] [CrossRef]
- Xu, B.; Sai-Anand, G.; Jeong, H.-M.; Kim, S.-W.; Kim, J.-S.; Kwon, J.-B.; Kang, S.-W. Improving Air-Stability and Performance of Bulk Heterojunction Polymer Solar Cells Using Solvent Engineered Hole Selective Interlayer. Materials 2018, 11, 1143. [Google Scholar] [CrossRef] [Green Version]
- Sai-Anand, G.; Dubey, A.; Gopalan, A.-I.; Venkatesan, S.; Ruban, S.; Reza, K.M.; Choi, J.; Lakhi, K.S.; Xu, B.; Qiao, Q.; et al. Additive assisted morphological optimization of photoactive layer in polymer solar cells. Sol. Energy Mater. Sol. Cells 2018, 182, 246–254. [Google Scholar] [CrossRef]
- Xu, B.; Sai-Anand, G.; Gopalan, A.-I.; Qiao, Q.; Kang, S.-W. Improving Photovoltaic Properties of P3HT:IC60BA through the Incorporation of Small Molecules. Polymers 2018, 10, 121. [Google Scholar]
- Gopalan, S.-A.; Gopalan, A.-I.; Vinu, A.; Lee, K.-P.; Kang, S.-W. A new optical-electrical integrated buffer layer design based on gold nanoparticles tethered thiol containing sulfonated polyaniline towards enhancement of solar cell performance. Sol. Energy Mater. Sol. Cells 2018, 174, 112–123. [Google Scholar] [CrossRef]
- Kang, B.-H.; Kim, J.-S.; Lee, J.-S.; Lee, S.-W.; Sai-Anand, G.; Jeong, H.-M.; Lee, S.-H.; Kwon, D.-H.; Kang, S.-W. Solution Processable CdSe/ZnS Quantum Dots Light-Emitting Diodes Using ZnO Nanocrystal as Electron Transport Layer. J. Nanosci. Nanotechnol. 2015, 15, 7416–7420. [Google Scholar] [CrossRef]
- Lee, S.-W.; Choi, K.-J.; Kang, B.-H.; Lee, J.-S.; Kim, S.-W.; Kwon, J.-B.; Gopalan, S.-A.; Bae, J.-H.; Kim, E.-S.; Kwon, D.-H.; et al. Low dark current and improved detectivity of hybrid ultraviolet photodetector based on carbon-quantum-dots/zinc-oxide-nanorod composites. Org. Electron. 2016, 39, 250–257. [Google Scholar] [CrossRef]
- Lee, S.-W.; Cha, S.-H.; Choi, K.-J.; Kang, B.-H.; Lee, J.-S.; Kim, S.-W.; Kim, J.-S.; Jeong, H.-M.; Gopalan, S.-A.; Kwon, D.-H.; et al. Low Dark-Current, High Current-Gain of PVK/ZnO Nanoparticles Composite-Based UV Photodetector by PN-Heterojunction Control. Sensors 2016, 16, 74. [Google Scholar]
- Jiang, Y.; Sai-Anand, G.; Xu, B.; Lee, J.-S.; Kim, S.-W.; Yeom, S.-H.; Bae, J.-H.; Kang, S.-W. Enhancing the Photovoltaic Performance of Polymer Solar Cells by Manipulating Photoactive/Metal Interface. J. Nanosci. Nanotechnol. 2017, 17, 8024–8030. [Google Scholar] [CrossRef]
- Saianand, G.; Gopalan, A.-I.; Lee, J.-C.; Sathish, C.; Gopalakrishnan, K.; Unni, G.E.; Shanbhag, D.; Dasireddy, V.D.B.C.; Yi, J.; Xi, S.; et al. Mixed Copper/Copper-Oxide Anchored Mesoporous Fullerene Nanohybrids as Superior Electrocatalysts toward Oxygen Reduction Reaction. Small 2020, 16, 1903937. [Google Scholar] [CrossRef]
- Karthikeyan, V.; Roy, V.A.L.; Gopalan, A.-I.; Saianand, G.; Kim, W.-J.; Kannan, V. A Comparative Evaluation of Physicochemical Properties and Photocatalytic Efficiencies of Cerium Oxide and Copper Oxide Nanofluids. Catalysts 2020, 10, 34. [Google Scholar]
- Bui, Q.-T.; Yu, I.-K.; Gopalan, A.I.; Saianand, G.; Kim, W.; Choi, S.-H. Facile Fabrication of Metal Oxide Based Catalytic Electrodes by AC Plasma Deposition and Electrochemical Detection of Hydrogen Peroxide. Catalysts 2019, 9, 888. [Google Scholar]
- Sridara, T.; Upan, J.; Saianand, G.; Tuantranont, A.; Karuwan, C.; Jakmunee, J. Non-Enzymatic Amperometric Glucose Sensor Based on Carbon Nanodots and Copper Oxide Nanocomposites Electrode. Sensors 2020, 20, 808. [Google Scholar]
- Lee, J.-C.; Gopalan, A.-I.; Saianand, G.; Lee, K.-P.; Kim, W.-J. Manganese and Graphene Included Titanium Dioxide Composite Nanowires: Fabrication, Characterization and Enhanced Photocatalytic Activities. Nanomaterials 2020, 10, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-C.; Gopalan, A.-I.; Sai-Anand, G.; Lee, K.-P.; Kim, W.-J. Preparation of Visible Light Photocatalytic Graphene Embedded Rutile Titanium(IV) Oxide Composite Nanowires and Enhanced NOx Removal. Catalysts 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-G.; Gopalan, A.-I.; Sai-Anand, G.; Kang, S.-W.; Lee, K.-P. New Heterojunction Titanium Dioxide Nanowire as Photocatalyst. J. Nanosci. Nanotechnol. 2015, 15, 7421–7425. [Google Scholar] [CrossRef]
- Haldorai, Y.; Hwang, S.-K.; Gopalan, A.-I.; Huh, Y.S.; Han, Y.-K.; Voit, W.; Sai-Anand, G.; Lee, K.-P. Direct electrochemistry of cytochrome c immobilized on titanium nitride/multi-walled carbon nanotube composite for amperometric nitrite biosensor. Biosens. Bioelectron. 2016, 79, 543–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrami, B.; Mabrouk, S.; Adhikari, N.; Elbohy, H.; Gurung, A.; Reza, K.M.; Pathak, R.; Chowdhury, A.H.; Saianand, G.; Yue, W.; et al. Nanoscale control of grain boundary potential barrier, dopant density and filled trap state density for higher efficiency perovskite solar cells. InfoMat 2020, 2, 409–423. [Google Scholar] [CrossRef] [Green Version]
- Sai-Anand, G.; Sivanesan, A.; Benzigar, M.R.; Singh, G.; Gopalan, A.-I.; Baskar, A.V.; Ilbeygi, H.; Ramadass, K.; Kambala, V.; Vinu, A. Recent Progress on the Sensing of Pathogenic Bacteria Using Advanced Nanostructures. Bull. Chem. Soc. Jpn. 2019, 92, 216–244. [Google Scholar] [CrossRef] [Green Version]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic degradation of VOCs on various commercial titanium dioxides: Impact of operating parameters on removal efficiency and by-products generation. Build. Environ. 2018, 138, 275–282. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Dong, X.a.; Zhang, W.; Sun, Y.; Li, J.; Cen, W.; Cui, Z.; Huang, H.; Dong, F. Visible-light-induced charge transfer pathway and photocatalysis mechanism on Bi semimetal@defective BiOBr hierarchical microspheres. J. Catal. 2018, 357, 41–50. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Cui, W.; Sun, Y.; Jiang, G.; Zhang, Y.; Huang, H.; Dong, F. Bismuth spheres assembled on graphene oxide: Directional charge transfer enhances plasmonic photocatalysis and in situ DRIFTS studies. Appl. Catal. B Environ. 2018, 221, 482–489. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Jiang, G.; Zhang, Y.; Huang, H.; Wu, Z.; Lee, S.C.; Dong, F. Unraveling the Mechanisms of Visible Light Photocatalytic NO Purification on Earth-Abundant Insulator-Based Core–Shell Heterojunctions. Environ. Sci. Technol. 2018, 52, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, W.; Dong, X.a.; Wang, H.; Dong, F. In situ FT-IR investigation on the reaction mechanism of visible light photocatalytic NO oxidation with defective g-C3N4. Sci. Bull. 2018, 63, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Anand, G.S.; Gopalan, A.I.; Kang, S.-W.; Lee, K.-P. Development of a surface plasmon assisted label-free calorimetric method for sensitive detection of mercury based on functionalized gold nanorods. J. Anal. At. Spectrom. 2013, 28, 488–498. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H.M. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Pérez-Lombard, L.; Ortiz, J.; Pout, C. A review on buildings energy consumption information. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Kuo, C.-F.J.; Lin, C.-H.; Hsu, M.-W.; Li, M.-H. Evaluation of intelligent green building policies in Taiwan—Using fuzzy analytic hierarchical process and fuzzy transformation matrix. Energy Build. 2017, 139, 146–159. [Google Scholar] [CrossRef]
- Younger, M.; Morrow-Almeida, H.R.; Vindigni, S.M.; Dannenberg, A.L. The Built Environment, Climate Change, and Health: Opportunities for Co-Benefits. Am. J. Prev. Med. 2008, 35, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Strini, A.; Cassese, S.; Schiavi, L. Measurement of benzene, toluene, ethylbenzene and o-xylene gas phase photodegradation by titanium dioxide dispersed in cementitious materials using a mixed flow reactor. Appl. Catal. B Environ. 2005, 61, 90–97. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; De Witte, B.; Beeldens, A.; Van Langenhove, H. Heterogeneous photocatalytic removal of toluene from air on building materials enriched with TiO2. Build. Environ. 2008, 43, 406–414. [Google Scholar] [CrossRef]
- Maggos, T.; Bartzis, J.G.; Liakou, M.; Gobin, C. Photocatalytic degradation of NOx gases using TiO2-containing paint: A real scale study. J. Hazard. Mater. 2007, 146, 668–673. [Google Scholar] [CrossRef]
- Maggos, T.; Plassais, A.; Bartzis, J.G.; Vasilakos, C.; Moussiopoulos, N.; Bonafous, L. Photocatalytic degradation of NOx in a pilot street canyon configuration using TiO2-mortar panels. Environ. Monit. Assess. 2008, 136, 35–44. [Google Scholar] [CrossRef]
- Binas, V.; Papadaki, D.; Maggos, T.; Katsanaki, A.; Kiriakidis, G. Study of innovative photocatalytic cement based coatings: The effect of supporting materials. Constr. Build. Mater. 2018, 168, 923–930. [Google Scholar] [CrossRef]
- Silvestre, J.; Silvestre, N.; de Brito, J. Review on concrete nanotechnology. Eur. J. Environ. Civ. Eng. 2016, 20, 455–485. [Google Scholar] [CrossRef]
- Nath, R.K.; Zain, M.F.M.; Jamil, M. An environment-friendly solution for indoor air purification by using renewable photocatalysts in concrete: A review. Renew. Sustain. Energy Rev. 2016, 62, 1184–1194. [Google Scholar] [CrossRef]
- Bastos, G.; Patiño-Barbeito, F.; Patiño-Cambeiro, F.; Armesto, J. Nano-Inclusions Applied in Cement-Matrix Composites: A Review. Materials (Basel) 2016, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Poon, C.-S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Perrin, M.L.; Verzijl, C.J.O.; Martin, C.A.; Shaikh, A.J.; Eelkema, R.; van Esch, J.H.; van Ruitenbeek, J.M.; Thijssen, J.M.; van der Zant, H.S.J.; Dulić, D. Large tunable image-charge effects in single-molecule junctions. Nat. Nanotechnol. 2013, 8, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Antizar-Ladislao, B.; Galil, N.I. Biofilm and colloidal biomass dynamics in a shallow sandy contaminated aquifer under in-situ remediation conditions. Int. Biodeterior. Biodegrad. 2010, 64, 331–338. [Google Scholar] [CrossRef]
- Cappelletti, G.; Fermo, P.; Camiloni, M. Smart hybrid coatings for natural stones conservation. Prog. Org. Coat. 2015, 78, 511–516. [Google Scholar] [CrossRef]
- Franzoni, E.; Fregni, A.; Gabrielli, R.; Graziani, G.; Sassoni, E. Compatibility of photocatalytic TiO2-based finishing for renders in architectural restoration: A preliminary study. Build. Environ. 2014, 80, 125–135. [Google Scholar] [CrossRef]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Smits, M.; Chan, C.k.; Tytgat, T.; Craeye, B.; Costarramone, N.; Lacombe, S.; Lenaerts, S. Photocatalytic degradation of soot deposition: Self-cleaning effect on titanium dioxide coated cementitious materials. Chem. Eng. J. 2013, 222, 411–418. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Licciulli, A.; Munafò, P. Self-cleaning materials on Architectural Heritage: Compatibility of photo-induced hydrophilicity of TiO2 coatings on stone surfaces. J. Cult. Herit. 2013, 14, 1–7. [Google Scholar] [CrossRef]
- Gherardi, F.; Colombo, A.; D’Arienzo, M.; Di Credico, B.; Goidanich, S.; Morazzoni, F.; Simonutti, R.; Toniolo, L. Efficient self-cleaning treatments for built heritage based on highly photo-active and well-dispersible TiO2 nanocrystals. Microchem. J. 2016, 126, 54–62. [Google Scholar] [CrossRef]
- Petronella, F.; Pagliarulo, A.; Striccoli, M.; Calia, A.; Lettieri, M.; Colangiuli, D.; Curri, M.L.; Comparelli, R. Colloidal Nanocrystalline Semiconductor Materials as Photocatalysts for Environmental Protection of Architectural Stone. Crystals 2017, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Calia, A.; Lettieri, M.; Masieri, M. Durability assessment of nanostructured TiO2 coatings applied on limestones to enhance building surface with self-cleaning ability. Build. Environ. 2016, 110, 1–10. [Google Scholar] [CrossRef]
- Lorenzo, G.; Enrico, Q.; Marco, D.O. Superfici autopulenti e biocide nel restauro archeologico di pietre e laterizi. Restauro Archeol. 2016, 24, 28–43. [Google Scholar] [CrossRef]
- La Russa, M.F.; Rovella, N.; Alvarez de Buergo, M.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coat. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Inkyo, M.; Tahara, T.; Iwaki, T.; Iskandar, F.; Hogan, C.J.; Okuyama, K. Experimental investigation of nanoparticle dispersion by beads milling with centrifugal bead separation. J. Colloid Interface Sci. 2006, 304, 535–540. [Google Scholar] [CrossRef]
- Miao, J.; Zhang, R.; Zhang, L. Photocatalytic degradations of three dyes with different chemical structures using ball-milled TiO2. Mater. Res. Bull. 2018, 97, 109–114. [Google Scholar] [CrossRef]
- He, R.; Huang, X.; Zhang, J.; Geng, Y.; Guo, H. Preparation and Evaluation of Exhaust-Purifying Cement Concrete Employing Titanium Dioxide. Materials 2019, 12, 2182. [Google Scholar] [CrossRef] [Green Version]
- Kusters, K.A.; Pratsinis, S.E.; Thoma, S.G.; Smith, D.M. Energy—Size reduction laws for ultrasonic fragmentation. Powder Technol. 1994, 80, 253–263. [Google Scholar] [CrossRef]
- Doktycz, S.; Suslick, K. Interparticle collisions driven by ultrasound. Science 1990, 247, 1067–1069. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Yan, Y.; Hu, Z.; Xiao, B. Investigation on preparation of pyrite tailings-based mineral admixture with photocatalytic activity. Constr. Build. Mater. 2017, 138, 26–34. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Plank, J.; Ruiz-Izuriaga, D.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Photocatalytically active coatings for cement and air lime mortars: Enhancement of the activity by incorporation of superplasticizers. Constr. Build. Mater. 2018, 162, 628–648. [Google Scholar] [CrossRef] [Green Version]
- Lowke, D.; Gehlen, C. The zeta potential of cement and additions in cementitious suspensions with high solid fraction. Cem. Concr. Res. 2017, 95, 195–204. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Cordoni, C.; Munafò, P. Self-cleaning and de-polluting stone surfaces: TiO2 nanoparticles for limestone. Constr. Build. Mater. 2012, 37, 51–57. [Google Scholar] [CrossRef]
- Motter, J.S.; Miranda, L.F.R.; Bernucci, L.L.B. Performance of Hot Mix Asphalt Concrete Produced with Coarse Recycled Concrete Aggregate. J. Mater. Civ. Eng. 2015, 27, 04015030. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kurtis, K.E. Influence of TiO2 Nanoparticles on Early C3S Hydration. J. Am. Ceram. Soc. 2010, 93, 3399–3405. [Google Scholar] [CrossRef]
- Koelsch, M.; Cassaignon, S.; Ta Thanh Minh, C.; Guillemoles, J.F.; Jolivet, J.P. Electrochemical comparative study of titania (anatase, brookite and rutile) nanoparticles synthesized in aqueous medium. Thin Solid Film. 2004, 451–452, 86–92. [Google Scholar] [CrossRef]
- Nolan, N.T.; Seery, M.K.; Pillai, S.C. Spectroscopic Investigation of the Anatase-to-Rutile Transformation of Sol−Gel-Synthesized TiO2 Photocatalysts. J. Phys. Chem. C 2009, 113, 16151–16157. [Google Scholar] [CrossRef]
- Cassar, L. Photocatalysis of Cementitious Materials: Clean Buildings and Clean Air. Mrs Bull. 2004, 29, 328–331. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Adormaa, B.B.; Darkwah, W.K.; Ao, Y. Oxygen vacancies of the TiO2 nano-based composite photocatalysts in visible light responsive photocatalysis. RSC Adv. 2018, 8, 33551–33563. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.; Ullah, R.; Butt, A.M.; Gohar, N.D. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef]
- Mittal, A.; Mari, B.; Sharma, S.; Kumari, V.; Maken, S.; Kumari, K.; Kumar, N. Non-metal modified TiO2: A step towards visible light photocatalysis. J. Mater. Sci. Mater. Electron. 2019, 30, 3186–3207. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.C.; Nadagouda, M.; Han, C.; O’Shea, K.; El-Sheikh, S.M.; Ismail, A.A.; Dionysiou, D.D. Visible light-sensitized S, N and C co-doped polymorphic TiO2 for photocatalytic destruction of microcystin-LR. Appl. Catal. B Environ. 2014, 144, 614–621. [Google Scholar] [CrossRef]
- Bessegato, G.G.; Cardoso, J.C.; Zanoni, M.V.B. Enhanced photoelectrocatalytic degradation of an acid dye with boron-doped TiO2 nanotube anodes. Catal. Today 2015, 240, 100–106. [Google Scholar] [CrossRef]
- Truppi, A.; Petronella, F.; Placido, T.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Visible-Light-Active TiO2-Based Hybrid Nanocatalysts for Environmental Applications. Catalysts 2017, 7, 100. [Google Scholar] [CrossRef]
- Medhi, R.; Marquez, M.D.; Lee, T.R. Visible-Light-Active Doped Metal Oxide Nanoparticles: Review of their Synthesis, Properties, and Applications. ACS Appl. Nano Mater. 2020, 3, 6156–6185. [Google Scholar] [CrossRef]
- Wang, S.; Ding, Z.; Chang, X.; Xu, J.; Wang, D.-H. Modified Nano-TiO2 Based Composites for Environmental Photocatalytic Applications. Catalysts 2020, 10, 759. [Google Scholar]
- Kumar, A.; Khan, M.; He, J.; Lo, I.M.C. Recent developments and challenges in practical application of visible–light–driven TiO2–based heterojunctions for PPCP degradation: A critical review. Water Res. 2020, 170, 115356. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-Light Active Titanium Dioxide Nanomaterials with Bactericidal Properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumdar, A.; Pal, A. Recent advancements in visible-light-assisted photocatalytic removal of aqueous pharmaceutical pollutants. Clean Technol. Environ. Policy 2020, 22, 11–42. [Google Scholar] [CrossRef]
- Higashimoto, S. Titanium-Dioxide-Based Visible-Light-Sensitive Photocatalysis: Mechanistic Insight and Applications. Catalysts 2019, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Laufs, S.; Burgeth, G.; Duttlinger, W.; Kurtenbach, R.; Maban, M.; Thomas, C.; Wiesen, P.; Kleffmann, J. Conversion of nitrogen oxides on commercial photocatalytic dispersion paints. Atmos. Environ. 2010, 44, 2341–2349. [Google Scholar] [CrossRef]
- National Institute for Occupational Safety and Health (NIOSH). NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97–140; US Government Printing Office: Washington, DC, USA, 1997.
- Zouzelka, R.; Rathousky, J. Photocatalytic abatement of NOx pollutants in the air using commercial functional coating with porous morphology. Appl. Catal. B Environ. 2017, 217, 466–476. [Google Scholar] [CrossRef]
- Lasek, J.; Yu, Y.-H.; Wu, J.C.S. Removal of NOx by photocatalytic processes. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 29–52. [Google Scholar] [CrossRef]
- Ehrlich, R. Effect of nitrogen dioxide on resistance to respiratory infection. Bacteriol. Rev. 1966, 30, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Poon, C.S.; Cheung, E. NO removal efficiency of photocatalytic paving blocks prepared with recycled materials. Constr. Build. Mater. 2007, 21, 1746–1753. [Google Scholar] [CrossRef]
- Sopyan, I.; Watanabe, M.; Murasawa, S.; Hashimoto, K.; Fujishima, A. An efficient TiO2 thin-film photocatalyst: Photocatalytic properties in gas-phase acetaldehyde degradation. J. Photochem. Photobiol. A Chem. 1996, 98, 79–86. [Google Scholar] [CrossRef]
- TioCem®—High Tech Cement for the Reduction of Air Pollutants. 2008. Available online: https://www.yumpu.com/en/document/view/22223710/tiocem-heidelbergcement (accessed on 10 September 2020).
- Calia, A.; Lettieri, M.; Masieri, M.; Pal, S.; Licciulli, A.; Arima, V. Limestones coated with photocatalytic TiO2 to enhance building surface with self-cleaning and depolluting abilities. J. Clean. Prod. 2017, 165, 1036–1047. [Google Scholar] [CrossRef]
- Lettieri, M.; Colangiuli, D.; Masieri, M.; Calia, A. Field performances of nanosized TiO2 coated limestone for a self-cleaning building surface in an urban environment. Build. Environ. 2019, 147, 506–516. [Google Scholar] [CrossRef]
- Marco, G.; Bo, X. Air Quality Legislation and Standards in the European Union: Background, Status and Public Participation. Adv. Clim. Chang. Res. 2013, 4, 50–59. [Google Scholar] [CrossRef]
- Baral, A.; Sen, S.; Roesler, J.R. Use phase assessment of photocatalytic cool pavements. J. Clean. Prod. 2018, 190, 722–728. [Google Scholar] [CrossRef]
- Mothes, F.; Ifang, S.; Gallus, M.; Golly, B.; Boréave, A.; Kurtenbach, R.; Kleffmann, J.; George, C.; Herrmann, H. Bed flow photoreactor experiments to assess the photocatalytic nitrogen oxides abatement under simulated atmospheric conditions. Appl. Catal. B Environ. 2018, 231, 161–172. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Atmospheric NOx removal: Study of cement mortars with iron- and vanadium-doped TiO2 as visible light–sensitive photocatalysts. Constr. Build. Mater. 2017, 149, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Seo, D.; Yun, T.S. NOx removal rate of photocatalytic cementitious materials with TiO2 in wet condition. Build. Environ. 2017, 112, 233–240. [Google Scholar] [CrossRef]
- Faraldos, M.; Kropp, R.; Anderson, M.A.; Sobolev, K. Photocatalytic hydrophobic concrete coatings to combat air pollution. Catal. Today 2016, 259, 228–236. [Google Scholar] [CrossRef]
- Lee, B.Y.; Jayapalan, A.R.; Bergin, M.H.; Kurtis, K.E. Photocatalytic cement exposed to nitrogen oxides: Effect of oxidation and binding. Cem. Concr. Res. 2014, 60, 30–36. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Chen, J.; Xia, M.; Wang, T.; Poon, C.S. Pathways of conversion of nitrogen oxides by nano TiO2 incorporated in cement-based materials. Build. Environ. 2018, 144, 412–418. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.-S. Photocatalytic activity of titanium dioxide modified concrete materials—Influence of utilizing recycled glass cullets as aggregates. J. Environ. Manag. 2009, 90, 3436–3442. [Google Scholar] [CrossRef]
- Dewi, K.; Khair, H.; Irsyad, M. Development of Green Pavement for Reducing Oxides of Nitrogen (NOx) in the Ambient Air. J. Eng. Technol. Sci. 2016, 48, 159–172. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Leng, Z.; Hüben, M.; Oeser, M.; Steinauer, B. Photocatalytic pavements with epoxy-bonded TiO2-containing spreading material. Constr. Build. Mater. 2016, 107, 44–51. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Navarro-Blasco, Í.; Fernández, J.M.; Alvarez, J.I. The Effect of TiO2 Doped Photocatalytic Nano-Additives on the Hydration and Microstructure of Portland and High Alumina Cements. Nanomaterials 2017, 7, 329. [Google Scholar] [CrossRef] [Green Version]
- Janus, M.; Bubacz, K.; Zatorska, J.; Kusiak-Nejman, E.; Czyżewski, A.; Morawski, A.W. NOx photocatalytic degradation on gypsum plates modified by TiO2-N, C photocatalysts. Pol. J. Chem. Technol. 2015, 17, 8–12. [Google Scholar] [CrossRef]
- Xu, M.; Bao, Y.; Wu, K.; Shi, H.; Guo, X.; Li, V.C. Multiscale investigation of tensile properties of a TiO2-doped Engineered Cementitious Composite. Constr. Build. Mater. 2019, 209, 485–491. [Google Scholar] [CrossRef]
- Truffier-Boutry, D.; Fiorentino, B.; Bartolomei, V.; Soulas, R.; Sicardy, O.; Benayad, A.; Damlencourt, J.F.; Pépin-Donat, B.; Lombard, C.; Gandolfo, A.; et al. Characterization of photocatalytic paints: A relationship between the photocatalytic properties—Release of nanoparticles and volatile organic compounds. Environ. Sci. Nano 2017, 4, 1998–2009. [Google Scholar] [CrossRef]
- Anantha-Iyengar, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.-P.; Whitcombe, M.J.; Lakshmi, D.; Sai-Anand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Bonnefond, A.; González, E.; Asua, J.M.; Leiza, J.R.; Ieva, E.; Brinati, G.; Carella, S.; Marrani, A.; Veneroni, A.; Kiwi, J.; et al. Stable Photocatalytic Paints Prepared from Hybrid Core-Shell Fluorinated/Acrylic/TiO2 Waterborne Dispersions. Crystals 2016, 6, 136. [Google Scholar] [CrossRef] [Green Version]
- Paolini, R.; Sleiman, M.; Pedeferri, M.; Diamanti, M.V. TiO2 alterations with natural aging: Unveiling the role of nitric acid on NIR reflectance. Sol. Energy Mater. Sol. Cells 2016, 157, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Manzhos, S.; Giorgi, G.; Yamashita, K. A Density Functional Tight Binding Study of Acetic Acid Adsorption on Crystalline and Amorphous Surfaces of Titania. Molecules 2015, 20, 3371–3388. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Maury-Ramirez, A.; Poon, C.S. Versatile photocatalytic functions of self-compacting architectural glass mortars and their inter-relationship. Mater. Des. 2015, 88, 1260–1268. [Google Scholar] [CrossRef]
- Gandolfo, A.; Bartolomei, V.; Gomez Alvarez, E.; Tlili, S.; Gligorovski, S.; Kleffmann, J.; Wortham, H. The effectiveness of indoor photocatalytic paints on NOx and HONO levels. Appl. Catal. B Environ. 2015, 166–167, 84–90. [Google Scholar] [CrossRef]
- Mendoza, C.; Valle, A.; Castellote, M.; Bahamonde, A.; Faraldos, M. TiO2 and TiO2–SiO2 coated cement: Comparison of mechanic and photocatalytic properties. Appl. Catal. B Environ. 2015, 178, 155–164. [Google Scholar] [CrossRef]
- Ângelo, J.; Andrade, L.; Mendes, A. Highly active photocatalytic paint for NOx abatement under real-outdoor conditions. Appl. Catal. A Gen. 2014, 484, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Luna, M.; Gatica, J.M.; Vidal, H.; Mosquera, M.J. Au-TiO2/SiO2 photocatalysts with NOx depolluting activity: Influence of gold particle size and loading. Chem. Eng. J. 2019, 368, 417–427. [Google Scholar] [CrossRef]
- Luna, M.; Gatica, J.M.; Vidal, H.; Mosquera, M.J. One-pot synthesis of Au/N-TiO2 photocatalysts for environmental applications: Enhancement of dyes and NOx photodegradation. Powder Technol. 2019, 355, 793–807. [Google Scholar] [CrossRef]
- Luna, M.; Gatica, J.M.; Vidal, H.; Mosquera, M.J. Use of Au/N-TiO2/SiO2 photocatalysts in building materials with NO depolluting activity. J. Clean. Prod. 2020, 243, 118633. [Google Scholar] [CrossRef]
- Lee, J.-S.; Yoon, N.-R.; Kang, B.-H.; Lee, S.-W.; Gopalan, S.-A.; Jeong, H.-M.; Lee, S.-H.; Kwon, D.-H.; Kang, S.-W. Response Characterization of a Fiber Optic Sensor Array with Dye-Coated Planar Waveguide for Detection of Volatile Organic Compounds. Sensors 2014, 14, 11659–11671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jedrychowski, W.; Perera, F.; Mrozek-Budzyn, D.; Mroz, E.; Flak, E.; Spengler, J.D.; Edwards, S.; Jacek, R.; Kaim, I.; Skolicki, Z. Gender differences in fetal growth of newborns exposed prenatally to airborne fine particulate matter. Environ. Res. 2009, 109, 447–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Srebric, J.; Li, X.; He, G. Performance of three air distribution systems in VOC removal from an area source. Build. Environ. 2004, 39, 1289–1299. [Google Scholar] [CrossRef]
- Weschler, C.J. Changes in indoor pollutants since the 1950s. Atmos. Environ. 2009, 43, 153–169. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.H.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of Indoor Volatile Organic Compounds via Photocatalytic Oxidation: A Short Review and Prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A.; Łomot, D.; Chernyayeva, O.; Lisovytskiy, D. A new photocatalytic tool in VOCs abatement: Effective synergetic combination of sonication and light for the synthesis of monometallic palladium-containing TiO2. Appl. Catal. B Environ. 2014, 147, 624–632. [Google Scholar] [CrossRef]
- Abbas, N.; Hussain, M.; Russo, N.; Saracco, G. Studies on the activity and deactivation of novel optimized TiO2 nanoparticles for the abatement of VOCs. Chem. Eng. J. 2011, 175, 330–340. [Google Scholar] [CrossRef]
- Yao, N.; Lun Yeung, K. Investigation of the performance of TiO2 photocatalytic coatings. Chem. Eng. J. 2011, 167, 13–21. [Google Scholar] [CrossRef]
- Freyria, F.S.; Compagnoni, M.; Ditaranto, N.; Rossetti, I.; Piumetti, M.; Ramis, G.; Bonelli, B. Pure and Fe-Doped Mesoporous Titania Catalyse the Oxidation of Acid Orange 7 by H2O2 under Different Illumination Conditions: Fe Doping Improves Photocatalytic Activity under Simulated Solar Light. Catalysts 2017, 7, 213. [Google Scholar] [CrossRef]
- Song, I.; Lee, J.; Lee, G.; Han, J.W.; Kim, D.H. Chemisorption of NH3 on Monomeric Vanadium Oxide Supported on Anatase TiO2: A Combined DRIFT and DFT Study. J. Phys. Chem. C 2018, 122, 16674–16682. [Google Scholar] [CrossRef]
- Balci Leinen, M.; Dede, D.; Khan, M.U.; Çağlayan, M.; Koçak, Y.; Demir, H.V.; Ozensoy, E. CdTe Quantum Dot-Functionalized P25 Titania Composite with Enhanced Photocatalytic NO2 Storage Selectivity under UV and Vis Irradiation. ACS Appl. Mater. Interfaces 2019, 11, 865–879. [Google Scholar] [CrossRef] [Green Version]
- Saeli, M.; Piccirillo, C.; Tobaldi, D.M.; Binions, R.; Castro, P.M.L.; Pullar, R.C. A sustainable replacement for TiO2 in photocatalyst construction materials: Hydroxyapatite-based photocatalytic additives, made from the valorisation of food wastes of marine origin. J. Clean. Prod. 2018, 193, 115–127. [Google Scholar] [CrossRef]
- Tawari, A.; Einicke, W.-D.; Gläser, R. Photocatalytic Oxidation of NO over Composites of Titanium Dioxide and Zeolite ZSM-5. Catalysts 2016, 6, 31. [Google Scholar] [CrossRef]
- Dinh, C.-T.; Hoogland, S.; Sargent, E.H. Spontaneous and Light-Driven Conversion of NOx on Oxide-Modified TiO2 Surfaces. Ind. Eng. Chem. Res. 2015, 54, 12750–12756. [Google Scholar] [CrossRef]
- Yamamoto, A.; Mizuno, Y.; Teramura, K.; Hosokawa, S.; Tanaka, T. Surface Ba species effective for photoassisted NOx storage over Ba-modified TiO2 photocatalysts. Appl. Catal. B Environ. 2016, 180, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.; Yu, J.; Gan, L.; Guo, F.; Phan, D.; Xu, G. Upgrading V2O5-WO3/TiO2 deNOx Catalyst with TiO2-SiO2 Support Prepared from Ti-Bearing Blast Furnace Slag. Catalysts 2016, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Jõgi, I.; Stamate, E.; Irimiea, C.; Schmidt, M.; Brandenburg, R.; Hołub, M.; Bonisławski, M.; Jakubowski, T.; Kääriäinen, M.-L.; Cameron, D.C. Comparison of direct and indirect plasma oxidation of NO combined with oxidation by catalyst. Fuel 2015, 144, 137–144. [Google Scholar] [CrossRef]
- Ma, J.; He, H.; Liu, F. Effect of Fe on the photocatalytic removal of NOx over visible light responsive Fe/TiO2 catalysts. Appl. Catal. B Environ. 2015, 179, 21–28. [Google Scholar] [CrossRef]
- Zhu, W.; Xiao, S.; Zhang, D.; Liu, P.; Zhou, H.; Dai, W.; Liu, F.; Li, H. Highly Efficient and Stable Au/CeO2–TiO2 Photocatalyst for Nitric Oxide Abatement: Potential Application in Flue Gas Treatment. Langmuir 2015, 31, 10822–10830. [Google Scholar] [CrossRef] [PubMed]
- Polat, M.; Soylu, A.M.; Erdogan, D.A.; Erguven, H.; Vovk, E.I.; Ozensoy, E. Influence of the sol–gel preparation method on the photocatalytic NO oxidation performance of TiO2/Al2O3 binary oxides. Catal. Today 2015, 241, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Estudillo-Wong, L.A.; Arce-Estrada, E.M.; Manzo-Robledo, A. Interaction of NO during cathodic polarization in alkaline conditions at the interface of Pt-nanostructures supported on C and TiO2-C. Electrochim. Acta 2014, 134, 100–106. [Google Scholar] [CrossRef]
- Giampiccolo, A.; Tobaldi, D.M.; Leonardi, S.G.; Murdoch, B.J.; Seabra, M.P.; Ansell, M.P.; Neri, G.; Ball, R.J. Sol gel graphene/TiO2 nanoparticles for the photocatalytic-assisted sensing and abatement of NO2. Appl. Catal. B Environ. 2019, 243, 183–194. [Google Scholar] [CrossRef]
- Trapalis, A.; Todorova, N.; Giannakopoulou, T.; Boukos, N.; Speliotis, T.; Dimotikali, D.; Yu, J. TiO2/graphene composite photocatalysts for NOx removal: A comparison of surfactant-stabilized graphene and reduced graphene oxide. Appl. Catal. B Environ. 2016, 180, 637–647. [Google Scholar] [CrossRef]
- Liao, L.; Heylen, S.; Vallaey, B.; Keulemans, M.; Lenaerts, S.; Roeffaers, M.B.J.; Martens, J.A. Photocatalytic carbon oxidation with nitric oxide. Appl. Catal. B Environ. 2015, 166–167, 374–380. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, P.; Wang, Z.; Rao, Y.; Cao, J.-j.; Pu, S.; Ho, W.; Lee, S.C. Protonated g-C3N4/Ti3+ self-doped TiO2 nanocomposite films: Room-temperature preparation, hydrophilicity, and application for photocatalytic NOx removal. Appl. Catal. B Environ. 2019, 240, 122–131. [Google Scholar] [CrossRef]
- Rozman, N.; Tobaldi, D.M.; Cvelbar, U.; Puliyalil, H.; Labrincha, J.A.; Legat, A.; Sever Škapin, A. Hydrothermal Synthesis of Rare-Earth Modified Titania: Influence on Phase Composition, Optical Properties, and Photocatalytic Activity. Materials 2019, 12, 713. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, S.; Szpoganicz, B.; Schultz, J.; Mangrich, A.S.; Hotza, D.; García, D.E.; Labrincha, J.A. Doped and undoped anatase-based plates obtained from paper templates for photocatalytic oxidation of NOX. Ceram. Int. 2016, 42, 12074–12083. [Google Scholar] [CrossRef]
- Jeon, K.-Y.; Kim, W.-J.; Lee, C.-J.; Gopalan, A.-I.; Lee, K.-P. Nanophase Changes in Nickel Doped Titania Composites by Thermal Treatment and Photocatalytic Destruction of NOx. J. Nanosci. Nanotechnol. 2015, 15, 7262–7271. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Piccirillo, C.; Rozman, N.; Pullar, R.C.; Seabra, M.P.; Škapin, A.S.; Castro, P.M.L.; Labrincha, J.A. Effects of Cu, Zn and Cu-Zn addition on the microstructure and antibacterial and photocatalytic functional properties of Cu-Zn modified TiO2 nano-heterostructures. J. Photochem. Photobiol. A Chem. 2016, 330, 44–54. [Google Scholar] [CrossRef]
- Lorencik, S.; Yu, Q.L.; Brouwers, H.J.H. Photocatalytic coating for indoor air purification: Synergetic effect of photocatalyst dosage and silica modification. Chem. Eng. J. 2016, 306, 942–952. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Pullar, R.C.; Gualtieri, A.F.; Otero-Irurueta, G.; Singh, M.K.; Seabra, M.P.; Labrincha, J.A. Nitrogen-modified nano-titania: True phase composition, microstructure and visible-light induced photocatalytic NOx abatement. J. Solid State Chem. 2015, 231, 87–100. [Google Scholar] [CrossRef]
- Yamamoto, A.; Mizuno, Y.; Teramura, K.; Hosokawa, S.; Tanaka, T. Noble-Metal-Free NOx Storage over Ba-Modified TiO2 Photocatalysts under UV-Light Irradiation at Low Temperatures. ACS Catal. 2015, 5, 2939–2943. [Google Scholar] [CrossRef]
- Folli, A.; Bloh, J.Z.; Armstrong, K.; Richards, E.; Murphy, D.M.; Lu, L.; Kiely, C.J.; Morgan, D.J.; Smith, R.I.; McLaughlin, A.C.; et al. Improving the Selectivity of Photocatalytic NOx Abatement through Improved O2 Reduction Pathways Using Ti0.909W0.091O2Nx Semiconductor Nanoparticles: From Characterization to Photocatalytic Performance. ACS Catal. 2018, 8, 6927–6938. [Google Scholar] [CrossRef]
- Chen, Y.; Tong, S.; Wang, J.; Peng, C.; Ge, M.; Xie, X.; Sun, J. Effect of Titanium Dioxide on Secondary Organic Aerosol Formation. Environ. Sci. Technol. 2018, 52, 11612–11620. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Li, W.; Zhong, L.; Zhang, C.; Fang, Q.; Chen, G. Effect of preferential exposure of anatase TiO2 {001} facets on the performance of Mn-Ce/TiO2 catalysts for low-temperature selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2019, 369, 26–34. [Google Scholar] [CrossRef]
- Cerro-Prada, E.; García-Salgado, S.; Quijano, M.Á.; Varela, F. Controlled Synthesis and Microstructural Properties of Sol-Gel TiO2 Nanoparticles for Photocatalytic Cement Composites. Nanomaterials 2019, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Poyraz, A.S.; Kuo, C.-H.; Miao, R.; Meng, Y.; Chen, S.-Y.; Jiang, T.; Wenos, C.; Suib, S.L. Crystalline Mixed Phase (Anatase/Rutile) Mesoporous Titanium Dioxides for Visible Light Photocatalytic Activity. Chem. Mater. 2015, 27, 6–17. [Google Scholar] [CrossRef]
- Shiraishi, F.; Maruoka, D.; Tanoue, Y. A better UV light and TiO2-PET sheet arrangement for enhancing photocatalytic decomposition of volatile organic compounds. Sep. Purif. Technol. 2017, 175, 185–193. [Google Scholar] [CrossRef]
- Morin, J.; Gandolfo, A.; Temime-Roussel, B.; Strekowski, R.; Brochard, G.; Bergé, V.; Gligorovski, S.; Wortham, H. Application of a mineral binder to reduce VOC emissions from indoor photocatalytic paints. Build. Environ. 2019, 156, 225–232. [Google Scholar] [CrossRef]
- Mull, B.; Möhlmann, L.; Wilke, O. Photocatalytic Degradation of Toluene, Butyl Acetate and Limonene under UV and Visible Light with Titanium Dioxide-Graphene Oxide as Photocatalyst. Environments 2017, 4, 9. [Google Scholar] [CrossRef]
- Shayegan, Z.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation of volatile organic compounds for indoor environment applications: Three different scaled setups. Chem. Eng. J. 2019, 357, 533–546. [Google Scholar] [CrossRef]
- Tian, M.-J.; Liao, F.; Ke, Q.-F.; Guo, Y.-J.; Guo, Y.-P. Synergetic effect of titanium dioxide ultralong nanofibers and activated carbon fibers on adsorption and photodegradation of toluene. Chem. Eng. J. 2017, 328, 962–976. [Google Scholar] [CrossRef]
- Cheng, Z.; Gu, Z.; Chen, J.; Yu, J.; Zhou, L. Synthesis, characterization, and photocatalytic activity of porous La–N–co-doped TiO2 nanotubes for gaseous chlorobenzene oxidation. J. Environ. Sci. 2016, 46, 203–213. [Google Scholar] [CrossRef]
- Enea, D.; Bellardita, M.; Scalisi, P.; Alaimo, G.; Palmisano, L. Effects of weathering on the performance of self-cleaning photocatalytic paints. Cem. Concr. Compos. 2019, 96, 77–86. [Google Scholar] [CrossRef]
- Blommaerts, N.; Asapu, R.; Claes, N.; Bals, S.; Lenaerts, S.; Verbruggen, S.W. Gas phase photocatalytic spiral reactor for fast and efficient pollutant degradation. Chem. Eng. J. 2017, 316, 850–856. [Google Scholar] [CrossRef]
- Zhu, W.; Koziel, J.A.; Maurer, D.L. Mitigation of Livestock Odors Using Black Light and a New Titanium Dioxide-Based Catalyst: Proof-of-Concept. Atmosphere 2017, 8, 103. [Google Scholar] [CrossRef] [Green Version]
- Toro, C.; Jobson, B.T.; Haselbach, L.; Shen, S.; Chung, S.H. Photoactive roadways: Determination of CO, NO and VOC uptake coefficients and photolabile side product yields on TiO2 treated asphalt and concrete. Atmos. Environ. 2016, 139, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Saeli, M.; Tobaldi, D.M.; Rozman, N.; Sever Škapin, A.; Labrincha, J.A.; Pullar, R.C. Photocatalytic nano-composite architectural lime mortar for degradation of urban pollutants under solar and visible (interior) light. Constr. Build. Mater. 2017, 152, 206–213. [Google Scholar] [CrossRef]
- Janus, M.; Zatorska, J.; Zając, K.; Kusiak-Nejman, E.; Czyżewski, A.; Morawski, A.W. Study of nitric oxide degradation properties of photoactive concrete containing nitrogen and/or carbon co-modified titanium dioxide–preliminary findings. Micro Nano Lett. 2016, 11, 231–235. [Google Scholar] [CrossRef]
- Law, K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. TiO2 photocatalysts and diamond electrodes. Electrochim. Acta 2000, 45, 4683–4690. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E. Superhydrophilic and Superwetting Surfaces: Definition and Mechanisms of Control. Langmuir 2010, 26, 18621–18623. [Google Scholar] [CrossRef]
- Liu, K.; Cao, M.; Fujishima, A.; Jiang, L. Bio-Inspired Titanium Dioxide Materials with Special Wettability and Their Applications. Chem. Rev. 2014, 114, 10044–10094. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, L. Bio-Inspired Self-Cleaning Surfaces. Annu. Rev. Mater. Res. 2012, 42, 231–263. [Google Scholar] [CrossRef]
- Fürstner, R.; Barthlott, W.; Neinhuis, C.; Walzel, P. Wetting and Self-Cleaning Properties of Artificial Superhydrophobic Surfaces. Langmuir 2005, 21, 956–961. [Google Scholar] [CrossRef]
- Liu, K.; Yao, X.; Jiang, L. Recent developments in bio-inspired special wettability. Chem. Soc. Rev. 2010, 39, 3240–3255. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, W.; Dong, C.; Sreeprasad, T.S.; Xia, Z. Biomimetic self-cleaning surfaces: Synthesis, mechanism and applications. J. R. Soc. Interface 2016, 13, 20160300. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, S.; Zhang, J.; Du, J.; Lee, H.J. Gibbs Free Energy of Liquid Drops on Conical Fibers. Langmuir 2011, 27, 11867–11872. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-J.; Strano, M.S.; Blankschtein, D. Wetting translucency of graphene. Nat. Mater. 2013, 12, 866–869. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef] [Green Version]
- Campbell, C.T.; Sauer, J. Introduction: Surface Chemistry of Oxides. Chem. Rev. 2013, 113, 3859–3862. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T. Surface Science Studies of the Photoactivation of TiO2New Photochemical Processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef]
- Thomas, A.G.; Syres, K.L. Adsorption of organic molecules on rutile TiO2 and anatase TiO2 single crystal surfaces. Chem. Soc. Rev. 2012, 41, 4207–4217. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Lang, X.; Ma, W.; Chen, C.; Ji, H.; Zhao, J. Selective Aerobic Oxidation Mediated by TiO2 Photocatalysis. Acc. Chem. Res. 2014, 47, 355–363. [Google Scholar] [CrossRef]
- Blossey, R. Self-cleaning surfaces—Virtual realities. Nat. Mater. 2003, 2, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Bai, C. Ascent of Nanoscience in China. Science 2005, 309, 61–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragesh, P.; Anand Ganesh, V.; Nair, S.V.; Nair, A.S. A review on ‘self-cleaning and multifunctional materials’. J. Mater. Chem. A 2014, 2, 14773–14797. [Google Scholar] [CrossRef]
- Luna, M.; Mosquera, M.J.; Vidal, H.; Gatica, J.M. Au-TiO2/SiO2 photocatalysts for building materials: Self-cleaning and de-polluting performance. Build. Environ. 2019, 164, 106347. [Google Scholar] [CrossRef]
- Truppi, A.; Luna, M.; Petronella, F.; Falcicchio, A.; Giannini, C.; Comparelli, R.; Mosquera, M.J. Photocatalytic Activity of TiO2/AuNRs–SiO2 Nanocomposites Applied to Building Materials. Coatings 2018, 8, 296. [Google Scholar] [CrossRef] [Green Version]
- Luna, M.; Delgado, J.J.; Gil, M.L.A.; Mosquera, M.J. TiO2-SiO2 Coatings with a Low Content of AuNPs for Producing Self-Cleaning Building Materials. Nanomaterials 2018, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Zhang, C.; Li, Q.; Zhang, W.; Cao, L.; Ye, J. Preparation of titanium dioxide nano particle modified photocatalytic self-cleaning concrete. J. Clean. Prod. 2015, 87, 762–765. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Peng, B.; Chai, L.; Liu, H. Self-cleaning performance of TiO2-coating cement materials prepared based on solidification/stabilization of electrolytic manganese residue. Constr. Build. Mater. 2016, 106, 236–242. [Google Scholar] [CrossRef]
- Janus, M.; Zatorska, J.; Czyżewski, A.; Bubacz, K.; Kusiak-Nejman, E.; Morawski, A.W. Self-cleaning properties of cement plates loaded with N,C-modified TiO2 photocatalysts. Appl. Surf. Sci. 2015, 330, 200–206. [Google Scholar] [CrossRef]
- Werle, A.P.; de Souza, M.L.; Loh, K.; Ando, R.; John, V.M. The performance of a self-cleaning cool cementitious surface. Energy Build. 2016, 114, 200–205. [Google Scholar] [CrossRef]
- Senff, L.; Tobaldi, D.M.; Lemes-Rachadel, P.; Labrincha, J.A.; Hotza, D. The influence of TiO2 and ZnO powder mixtures on photocatalytic activity and rheological behavior of cement pastes. Constr. Build. Mater. 2014, 65, 191–200. [Google Scholar] [CrossRef]
- Ono, S.; Kishikawa, N.; Kawase, S.; Hayashi, T.; Asano, N. Low-Cost Preparation Method for Anti-Dirt Coating on Concrete Block Using Titanium Oxide Photocatalytic Powder. In Proceedings of the 12th Pacific Rim Conference on Ceramic and Glass Technology, Waikoloa, HI, USA, 21–26 May 2017; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 267–277. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, T.; Su, W.; Yang, B.; Zhang, Y.; Yang, Z. Photocatalytic NOx abatement and self-cleaning performance of cementitious composites with g-C3N4 nanosheets under visible light. Constr. Build. Mater. 2019, 225, 120–131. [Google Scholar] [CrossRef]
- Amor, F.; Diouri, A.; Ellouzi, I.; Ouanji, F. Development of Zn-Al-Ti mixed oxides-modified cement phases for surface photocatalytic performance. Case Stud. Constr. Mater. 2018, 9, e00209. [Google Scholar] [CrossRef]
- Wang, D.; Hou, P.; Zhang, L.; Xie, N.; Yang, P.; Cheng, X. Photocatalytic activities and chemically-bonded mechanism of SiO2@TiO2 nanocomposites coated cement-based materials. Mater. Res. Bull. 2018, 102, 262–268. [Google Scholar] [CrossRef]
- Zhao, A.; Yang, J.; Yang, E.-H. Self-cleaning engineered cementitious composites. Cem. Concr. Compos. 2015, 64, 74–83. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Rodriguez-Garcia, J.R.; Castillo, A.; Castellote, M. Characteristics and efficiency of photocatalytic cementitious materials: Type of binder, roughness and microstructure. Cem. Concr. Res. 2015, 71, 124–131. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Paolini, R.; Rossini, M.; Aslan, A.B.; Zinzi, M.; Poli, T.; Pedeferri, M.P. Long term self-cleaning and photocatalytic performance of anatase added mortars exposed to the urban environment. Constr. Build. Mater. 2015, 96, 270–278. [Google Scholar] [CrossRef]
- García, L.D.; Pastor, J.M.; Peña, J. Self cleaning and depolluting glass reinforced concrete panels: Fabrication, optimization and durability evaluation. Constr. Build. Mater. 2018, 162, 9–19. [Google Scholar] [CrossRef]
- Smits, M.; Huygh, D.; Craeye, B.; Lenaerts, S. Effect of process parameters on the photocatalytic soot degradation on self-cleaning cementitious materials. Catal. Today 2014, 230, 250–255. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Maury-Ramirez, A.; Poon, C.S. Photocatalytic activities of titanium dioxide incorporated architectural mortars: Effects of weathering and activation light. Build. Environ. 2015, 94, 395–402. [Google Scholar] [CrossRef]
- Vulic, T.; Rudic, O.; Vucetic, S.; Lazar, D.; Ranogajec, J. Photocatalytic activity and stability of TiO2/ZnAl layered double hydroxide based coatings on mortar substrates. Cem. Concr. Compos. 2015, 58, 50–58. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Dionísio, A. Self-cleaning property of mortars with TiO2 addition using real diesel exhaust soot. J. Clean. Prod. 2017, 161, 850–859. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Maury-Ramirez, A.; Poon, C.S. Self-cleaning ability of titanium dioxide clear paint coated architectural mortar and its potential in field application. J. Clean. Prod. 2016, 112, 3583–3588. [Google Scholar] [CrossRef]
- Sikora, P.; Cendrowski, K.; Markowska-Szczupak, A.; Horszczaruk, E.; Mijowska, E. The effects of silica/titania nanocomposite on the mechanical and bactericidal properties of cement mortars. Constr. Build. Mater. 2017, 150, 738–746. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Miller, A.Z.; Pozo-Antonio, J.S.; González-Pérez, J.A.; Jiménez-Morillo, N.T.; Dionisio, A. Assessing the effects of UVA photocatalysis on soot-coated TiO2-containing mortars. Sci. Total Environ. 2017, 605–606, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Laplaza, A.; Jimenez-Relinque, E.; Campos, J.; Castellote, M. Photocatalytic behavior of colored mortars containing TiO2 and iron oxide based pigments. Constr. Build. Mater. 2017, 144, 300–310. [Google Scholar] [CrossRef]
- Zanfir, A.-V.; Voicu, G.; Bădănoiu, A.-I.; Gogan, D.; Oprea, O.; Vasile, E. Synthesis and characterization of titania-silica fume composites and their influence on the strength of self-cleaning mortar. Compos. Part B Eng. 2018, 140, 157–163. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Castellote, M. Quick assessment of the photocatalytic activity of TiO2 construction materials by nitroblue tetrazolium (NBT) ink. Constr. Build. Mater. 2019, 214, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Lu, C.; Xiong, J. Self-cleaning and depollution of fiber reinforced cement materials modified by neutral TiO2/SiO2 hydrosol photoactive coatings. Appl. Surf. Sci. 2014, 298, 19–25. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, G.; Zhang, F.; Li, Q.; Zhao, T.; Zhu, X. Orthogonal experimentation for optimization of TiO2 nanoparticles hydrothermal synthesis and photocatalytic property of a TiO2/concrete composite. RSC Adv. 2015, 5, 6071–6078. [Google Scholar] [CrossRef]
- Delnavaz, M.; Ayati, B.; Ganjidoust, H.; Sanjabi, S. Application of concrete surfaces as novel substrate for immobilization of TiO2 nano powder in photocatalytic treatment of phenolic water. J. Environ. Health Sci. Eng. 2015, 13, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-J.; Yoon, Y.-S.; Yang, K.-H.; Kwon, S.-J. Durability and purification performance of concrete impregnated with silicate and sprayed with photocatalytic TiO2. Constr. Build. Mater. 2019, 199, 106–114. [Google Scholar] [CrossRef]
- Koli, V.B.; Mavengere, S.; Kim, J.-S. An efficient one-pot N doped TiO2-SiO2 synthesis and its application for photocatalytic concrete. Appl. Surf. Sci. 2019, 491, 60–66. [Google Scholar] [CrossRef]
- García Calvo, J.L.; Carballosa, P.; Castillo, A.; Revuelta, D.; Gutiérrez, J.P.; Castellote, M. Expansive concretes with photocatalytic activity for pavements: Enhanced performance and modifications of the expansive hydrates composition. Constr. Build. Mater. 2019, 218, 394–403. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Martínez-Ramírez, S.; Viles, H.A. Efficiency and durability of a self-cleaning coating on concrete and stones under both natural and artificial ageing trials. Appl. Surf. Sci. 2018, 433, 312–320. [Google Scholar] [CrossRef]
- Jalal, M.; Fathi, M.; Farzad, M. Effects of fly ash and TiO2 nanoparticles on rheological, mechanical, microstructural and thermal properties of high strength self compacting concrete. Mech. Mater. 2013, 61, 11–27. [Google Scholar] [CrossRef]
- Sun, R.-D.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Decomposition of gas-phase octamethyltrisiloxane on TiO2 thin film photocatalysts—Catalytic activity, deactivation, and regeneration. J. Photochem. Photobiol. A Chem. 2003, 154, 203–209. [Google Scholar] [CrossRef]
- Méndez-Román, R.; Cardona-Martínez, N. Relationship between the formation of surface species and catalyst deactivation during the gas-phase photocatalytic oxidation of toluene. Catal. Today 1998, 40, 353–365. [Google Scholar] [CrossRef]
- Cao, L.; Gao, Z.; Suib, S.L.; Obee, T.N.; Hay, S.O.; Freihaut, J.D. Photocatalytic Oxidation of Toluene on Nanoscale TiO2 Catalysts: Studies of Deactivation and Regeneration. J. Catal. 2000, 196, 253–261. [Google Scholar] [CrossRef]
- Piera, E.; Ayllón, J.A.; Doménech, X.; Peral, J. TiO2 deactivation during gas-phase photocatalytic oxidation of ethanol. Catal. Today 2002, 76, 259–270. [Google Scholar] [CrossRef]
- Marzo, T.; Pratesi, A.; Cirri, D.; Pillozzi, S.; Petroni, G.; Guerri, A.; Arcangeli, A.; Messori, L.; Gabbiani, C. Chlorido and bromido oxaliplatin analogues as potential agents for CRC treatment: Solution behavior, protein binding and cytotoxicity evaluation. Inorg. Chim. Acta 2018, 470, 318–324. [Google Scholar] [CrossRef]
- Peral, J.; Ollis, D.F. TiO2 photocatalyst deactivation by gas-phase oxidation of heteroatom organics. J. Mol. Catal. A Chem. 1997, 115, 347–354. [Google Scholar] [CrossRef]
- Van Driel, B.A.; Wezendonk, T.A.; van den Berg, K.J.; Kooyman, P.J.; Gascon, J.; Dik, J. Determination of early warning signs for photocatalytic degradation of titanium white oil paints by means of surface analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 172, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samain, L.; Silversmit, G.; Sanyova, J.; Vekemans, B.; Salomon, H.; Gilbert, B.; Grandjean, F.; Long, G.J.; Hermann, R.P.; Vincze, L.; et al. Fading of modern Prussian blue pigments in linseed oil medium. J. Anal. At. Spectrom. 2011, 26, 930–941. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Torgal, F.; Jalali, S. Nanotechnology: Advantages and drawbacks in the field of construction and building materials. Constr. Build. Mater. 2011, 25, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Shandilya, N.; Le Bihan, O.; Bressot, C.; Morgeneyer, M. Emission of Titanium Dioxide Nanoparticles from Building Materials to the Environment by Wear and Weather. Environ. Sci. Technol. 2015, 49, 2163–2170. [Google Scholar] [CrossRef]
- Vorbau, M.; Hillemann, L.; Stintz, M. Method for the characterization of the abrasion induced nanoparticle release into air from surface coatings. J. Aerosol Sci. 2009, 40, 209–217. [Google Scholar] [CrossRef]
- Le Bihan, O.; Shandilya, N.; Gheerardyn, L.; Guillon, O.; Dore, E.; Morgeneyer, M. Investigation of the release of particles from a nanocoated product. Adv. Nanopart. 2013, 2, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Kebede, M.A.; Varner, M.E.; Scharko, N.K.; Gerber, R.B.; Raff, J.D. Photooxidation of Ammonia on TiO2 as a Source of NO and NO2 under Atmospheric Conditions. J. Am. Chem. Soc. 2013, 135, 8606–8615. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Li, Z.; Zhang, L.; Zeng, S.; Yu, X.; Han, B.; Ou, J. Reactive powder concrete reinforced with nano SiO2-coated TiO2. Constr. Build. Mater. 2017, 148, 104–112. [Google Scholar] [CrossRef]
- Marugán, J.; López-Muñoz, M.-J.; van Grieken, R.; Aguado, J. Photocatalytic Decolorization and Mineralization of Dyes with Nanocrystalline TiO2/SiO2 Materials. Ind. Eng. Chem. Res. 2007, 46, 7605–7610. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Zheng, X.; Bondarenko, V.; Fava, G.; Ruello, M.L. Paving the Way for A Sustainable and Efficient SiO2/TiO2 Photocatalytic Composite. Environments 2019, 6, 87. [Google Scholar] [CrossRef] [Green Version]


















| VOCs | Potential Sources |
|---|---|
| Formaldehyde | Pesticides, flooring/insulating/wood materials, coatings, paints, etc. |
| Acetaldehyde | Flooring/wood materials, etc. |
| Naphthalene | Insulating/mixed materials, etc. |
| Chloroform | Glues, pesticides, etc. |
| Ethylbenzene | Adhesive, paints, furniture, etc. |
| Dichlorobenzene | Wood/ceiling materials, etc. |
| Carbon tetrachloride | Paints, coatings, industrial cleaning agents, etc. |
| Toluene | Pesticides, flooring/insulating/wood materials, adhesives, paintings, etc. |
| Others (ketones, esters) | Perfumes, paints, adhesives, plastics, resins, disinfectants, etc. |
| Modification Type | Specific Example | Methodology | Remarks on NOx Removal | Reference |
|---|---|---|---|---|
| Composites | Vanadium/TiO2 | Functional theory calculations combined with in-situ diffuse reflectance infrared Fourier transform | Selective photooxidation-stable formation of V/TiO2 surface | [146] |
| P25 (titania) functionalized with thioglycolic acid (TGA)-capped CdTe colloidal quantum dots (QDs) | Chemical methods | TGA, CdTe, and titania components in the TGA-CdTe/P25 composite | [147] | |
| Hydroxyapatite (HAp)/beta-tricalcium phosphate/TiO2 | Intermixing and a post-curing coating procedure | Active sites are available on the surface of the material leading to greater NOx oxidation | [148] | |
| Composites of TiO2 (Hombikat, P25, sol-gel synthesis) and zeolite ZSM-5 | Sol-gel and solid-state dispersion | Well-dispersed TiO2 particles over the ZSM-5 crystals caused good NOx removal | [149] | |
| TiO2 covered with a thin layer of an alumina–silica | Commercial | Coating TiO2 with Al2O3 and SiO2 dramatically increases the conversion of NO2 to NO on TiO2 | [150] | |
| TiO2 photocatalyst with Ba species | Impregnation | Ba–Ti mixed oxide phase was formed-the surface of amorphous Ba–Ti mixed oxides could store NO3− more densely than the TiO2 surface | [151] | |
| TiO2–SiO2 mixed oxides | Blast furnace, Chemical method, and calcination | High activity in selective catalytic reduction, excellent stability, and low preparation catalyst cost | [152] | |
| TiO2 and ZnO | Plasma treatment | Longer reaction times for efficient oxidation of NOx | [153] | |
| Fe/TiO2 | Co-precipitation | Fe increased the electron-hole separation efficiency because Fe3+ incorporated into TiO2 can act as an electron-trapped agent | [154] | |
| Au/CeO2−TiO2 | Chemical method | Au−Ce3+ interface formed and served as an anchoring site of the O2 molecule. Then more adsorbed oxygen could react with photogenerated electrons on TiO2 surfaces to produce more superoxide radicals for NO oxidation | [155] | |
| TiO2/Al2O3binary oxide | Sol-gel synthesis protocols | Alumina domains can be utilized as active NOx capturing sites that can significantly eliminate the release of toxic NO | [156] | |
| TiO2-Carbon | Chemical method | Reduction of NO towards N2 by proton adsorption-promotion on Pt-Pt sites or by selective-secondary sites (as titanium dioxide) | [157] | |
| TiO2/G hybrids | Sol-gel | Interaction between TiO2 nanoparticles and graphene sheets, acting as electron traps. | [158] | |
| G included pure rutile TiO2 nanowire | Electrospinning, sol-gel, and calcination | The inclusion of G into TiO2 nanostructures enhances the visible light photoactivity | [33] | |
| TiO2/G | Solvothermal process | Favorable NO2 adsorption on graphene through an interaction between the NO2 molecules and the conjugated system in graphene sheets | [159] | |
| TiO2/Printex U (a famous model carbon black) | Coating | Reduction of NO to nitrogen, achieving DeNOx without any reducing agent | [160] | |
| Doping | Ti3+ self-doped TiO2 NPs | Spray-coating | Surface oxygen vacancies caused by the diffusion of photo-generated holes play significant roles-Ti4+ is reduced to Ti3+, dissociating water molecules and facilitating the adsorption of −OH species on surfaces-photoinduced hydrophilicity | [161] |
| Modified with Ce, La, and Gd | Chemical method (gel precipitation) and hydrothermal treatment | The addition of rare earth elements to hydrothermal processing slowed the anatase-to-rutile and brookite-to-rutile transformation-grain boundary pinning effect | [162] | |
| TiO2 plates doped with Ba, La | Calcination | Doped with 5%-Zr4+ and calcined at 800 °C, resulted in 100% degradation of NOx as compared to commercial P25. The addition of Zr4+ caused a decrease in crystalline sizes of both anatase and rutile forms causing an increase in surface area | [163] | |
| Ni doping | Thermal treatment | 6% weight Nickel doped KA100 and annealed at 1000 °C showed excellent NOx removal. Transformation into 100% rutile phase and size effects played roles in photoactivities. | [164] | |
| Cu, Zn, and Cu-Zn | Thermal treatment | 0.25 Cu/0.75 Zn-Ti450 (Cu:Zn molar ratio equal to 1:3) was the best performing specimen in degrading NOx | [165] | |
| Carbon-doped titanium dioxide and nano-silica | Coating and UV-pre treatment | Highest NO degradation is achieved by the two-layered coatings comprised of carbon-doped TiO2 and silica | [166] | |
| Nitrogen modified TiO2 | Sol-gel green synthetic method | Heated at 450 °C possessed excellent photocatalytic activity under visible white-light (indoor artificial lighting)-double PCA than P25 TiO2 NPs. | [167] | |
| Barium-modified titanium dioxide | Impregnation method | BaO species, which was generated from the decomposition of Ba(NO3) 2, works as a NOx storage material | [168] | |
| Nanostructuring and physical modifications | The electronic crystallographic structural relationship for Ti0.909W0.091O2Nx | Mixing and calcination | Oxygen vacancy acts as an electron trapping center for conduction-band electrons and reduction of NO | [169] |
| TiO2 to detect secondary aerosol pollutant formation | Seed via atomization | Reactive carbonyl compounds caused by the photo-degradation role of TiO2 lead to the suppression of C formation | [170] | |
| Titanium dioxide nanosheets and anatase titanium dioxide nanospindles with highly exposed (001) facets | Hydrothermal and faceted engineering | Preferential exposure of (001) facets of TiO2 had a positive effect on increasing the specific surface area of the catalysts | [171] | |
| Size controlled nanoparticles | Controlled annealing | Mortar mixtures with only 1 wt.% TiO2 resulted in NOx degradation rates close to 80% | [172] | |
| Crystalline mixed-phase (Anatase/Rutile) mesoporous | Calcination at temperatures (600–800 °C, 1 °C/min) followed by a second heat treatment | A synergetic effect between anatase and rutile | [173] |
| Type of Building Material | Pristine/ Modified TiO2 | Preparation Method | Testing Mode | Salient Results | Ref. |
|---|---|---|---|---|---|
| Cement paste | Pristine TiO2 | Mixing with cement paste (0.5~7.5% by weight of cement) | Photo-degradation of methylene blue (MB) under UV light | Photo-degradation ratio by 9.5% (0.5% for the plain) | [211] |
| Cement paste | Modified TiO2 | Sol-gel dip-coating on cement paste surface | Photo-degradation of methyl orange (MO) under UV light | MO degradation efficiency by 90% in 2 h | [212] |
| Cement paste | Modified TiO2 | Mixing with cement paste (1%, 5% and 10% by weight of cement) | RR198 dye degradation under UV light | RR198 dye degradation rate by 60% | [213] |
| Cement paste | Pristine TiO2 | Mixed with cement paste (5% and 30% by weight of cement) | Congo Red (CR) dye degradation under a UV lamp and visible lamp | Absorbance decreases by 94% in 5 h using P25 | [214] |
| Cement paste | Pristine TiO2 | Mixed with cement paste | Photo-degradation of MB under UV light | Photo-degradation ratio by 100% in 3 h | [215] |
| Cement paste | Pristine TiO2 | Coating on a cement paste substrate | Photo-degradation of MB under UV light | Performance similar to the reference material P25 in 2.5 h | [216] |
| Cement paste | Pristine TiO2 | Mixed with cement paste (2% by weight of cement) | Photo-degradation of Rhodamine B (RhB) under UV irradiation | Photo-degradation rate by 90% in 60 min | [217] |
| Cement paste | Modified TiO2 | Mixed with clinker | Photo-degradation of MB under UV light at the age of 6 months | Photo-degradation rate by 25% in 4 h | [218] |
| Cement paste | Modified TiO2 | Spraying TiO2 sols on cement paste | Photo-degradation of RhB under UV irradiation | Photo-degradation rate by 65% in 9 h | [219] |
| Mortar | Pristine TiO2 | Mixed with cement (0.5~10% by weight of cement) | Reflectance recovery subject to artificial UV or direct sunlight irradiation | Reflectance recovery by 85% in 8 h under UV irradiation | [220] |
| Mortar | Pristine TiO2 | Mixed with cement (2% by weight of cement) | Discoloration of organic dyes (RhB and MB) under UV irradiation | Color change by 40~60% in 24 h under UV irradiation | [221] |
| Mortar | Pristine TiO2 | Mixed with Mortar | Monitoring the variations in color under sunlight | After aging, photoactive samples showed limited color variation | [222] |
| Mortar | Pristine TiO2 | Mixed with Mortar (2.5~10%) | Photo-degradation of Rh Bunder daylight lamp | RhB dye degradation rate by 85% in 24 h | [223] |
| Mortar | Pristine TiO2 | Coating TiO2 dispersed in ethanol on the mortar surface. | Photo-degradation of soot concentration under UV light | Photo-degradation ratio by 70% | [224] |
| Mortar | Pristine TiO2 | TiO2 intermixing and coating | Photo-degradation of Rh Bunder visible light | RhB removal over 50% | [225] |
| Mortar | Modified TiO2 | Spraying TiO2 suspension on mortar | Photo-degradation of RhB under UV irradiation | RhB removal by 80% in 3.5 h | [226] |
| Mortar | Pristine TiO2 | Mixed with mortar (1~5% by weight of a mixture) | Photo-degradation of diesel exhaust soot under UV light and sunlight | Slightly higher degradation of organic matter on the surface for the mortar with higher TiO2 content | [227] |
| Mortar | Pristine TiO2 | Coated on mortar substrate and mixed with mortar | Photo-degradation of RhB under UV irradiation | RhB removal by 65% after weathering | [228] |
| Mortar | Modified TiO2 | Mixed with mortar (3% by weight of mortar) | Photo-degradation of RhB under mercury lamp | RhB removal by 100% within 1 h | [229] |
| Mortar | Pristine TiO2 | Mixed with mortar (2.5% and 5% by weight of mortar) | Photo-degradation of soot concentration under UV light | Promotion of self-cleaning effect for TiO2-containing mortars after UVA irradiation. | [230] |
| Mortar | Pristine TiO2 | Use of ready-mixed mortar containing TiO2 | Photo-degradation of RhB under UV irradiation | RhB discoloration rate by 55% in 26 h | [231] |
| Mortar | Pristine TiO2 | Spraying TiO2 sols on mortar surface | Photo-degradation of Rh Bunder fluorescent lamp | RhB photo-degradation rate by 100% in 5 days | [130] |
| Mortar | Pristine TiO2 | Coating on mortar surface | Photo-degradation of RhB under UV irradiation | RhB removal by 65% in 26 h | [128] |
| Mortar | Modified TiO2 | Mixed with mortar (3% by weight of cement) | Photo-degradation of MB under UV light | Photo-degradation rate by 35% in 4 h | [232] |
| Mortar | Pristine TiO2 | Mixed with mortar (2% by weight of cement) | Nitroblue tetrazolium (NBT) ink color change under UV light | NBT testing takes substantially less time (10 min) than conventional photocatalytic activity tests | [233] |
| Mortar | Modified TiO2 | Dip-coating of TiO2/SiO2 sol on mortar surface | Photo-degradation of RhB under UV irradiation | Photo-degradation rate by 90% in 5 h | [234] |
| Concrete | Modified TiO2 | Spraying TiO2 suspension on a porous concrete | Photo-degradation of MO under UV light | Photo-degradation ratio by 35% in 3 h | [235] |
| Concrete | Pristine TiO2 | Slurry method (SM) Cement mixed method (CMM) Epoxy sealer method (ESM) Waterproof sealer method (WSM) | Phenol removal efficiency under UV light | Phenol removal ratio by 85% in 4 h (ESM) | [236] |
| Concrete | Pristine TiO2 and Modified TiO2 | Spray and dip coating | Photo-degradation of MB under UV light | MB degradation by 5.2 × 10−4 pixels−1 | [114] |
| Concrete | Pristine TiO2 | Spraying TiO2 suspension on concrete | Photo-degradation of MO under UV light | MO degradation efficiency by 100% in 2 h | [237] |
| Concrete | Modified TiO2 | Coating on a concrete substrate | Photo-degradation of MB under UV light | Photo-degradation ratio by 100% in 5 h | [238] |
| Concrete | Pristine TiO2 | Mixed with concrete (3.5% by weight of cement) | Photo-degradation of MB under UV light | Photo-degradation ratio by 82% in 26 h | [239] |
| Concrete | Modified TiO2 | Spraying TiO2 sols on the concrete surface | Photo-degradation of Rh Bunder natural condition | RhB photo-degradation rate by 30% in 24 h (for the specimen at the age of 2000 h) | [240] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gopalan, A.-I.; Lee, J.-C.; Saianand, G.; Lee, K.-P.; Sonar, P.; Dharmarajan, R.; Hou, Y.-l.; Ann, K.-Y.; Kannan, V.; Kim, W.-J. Recent Progress in the Abatement of Hazardous Pollutants Using Photocatalytic TiO2-Based Building Materials. Nanomaterials 2020, 10, 1854. https://doi.org/10.3390/nano10091854
Gopalan A-I, Lee J-C, Saianand G, Lee K-P, Sonar P, Dharmarajan R, Hou Y-l, Ann K-Y, Kannan V, Kim W-J. Recent Progress in the Abatement of Hazardous Pollutants Using Photocatalytic TiO2-Based Building Materials. Nanomaterials. 2020; 10(9):1854. https://doi.org/10.3390/nano10091854
Chicago/Turabian StyleGopalan, Anantha-Iyengar, Jun-Cheol Lee, Gopalan Saianand, Kwang-Pill Lee, Prashant Sonar, Rajarathnam Dharmarajan, Yao-long Hou, Ki-Yong Ann, Venkatramanan Kannan, and Wha-Jung Kim. 2020. "Recent Progress in the Abatement of Hazardous Pollutants Using Photocatalytic TiO2-Based Building Materials" Nanomaterials 10, no. 9: 1854. https://doi.org/10.3390/nano10091854