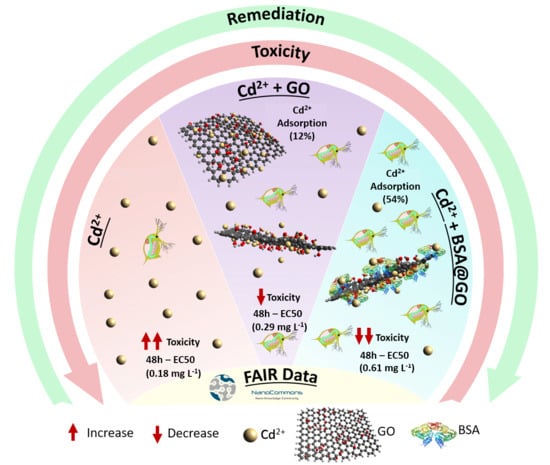
Effect of the Albumin Corona on the Toxicity of Combined Graphene Oxide and Cadmium to Daphnia magna and Integration of the Datasets into the NanoCommons Knowledge Base
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Management
2.2. Synthesis of the Graphene Oxide
2.3. Characterisation of Graphene Oxide
2.4. Preparation and Characterisation of BSA@GO Material
2.5. Dispersion Stability Studies
2.6. Cadmium Adsorption Experiments
2.7. Toxicity Assays with Daphnia Magna
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.B.; et al. Safety assessment of graphene-based materials: Focus on human health and the environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.J.; Silveira, C.P.; Martinez, D.S.T.; Filho, A.G.S.; Romero, F.V.; Fonseca, L.C.; Tasic, L.; Alves, O.L.; Durán, N. Topography-driven bionano-interactions on colloidal silica nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 3437–3447. [Google Scholar] [CrossRef]
- Durán, N.; Silveira, C.P.; Durán, M.; Martinez, D.S.T. Silver nanoparticle protein corona and toxicity: A mini-review. J. Nanobiotechnol. 2015, 13, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, L.C.; De Araújo, M.M.; De Moraes, A.C.M.; Da Silva, D.S.; Ferreira, A.G.; Franqui, L.S.; Martinez, D.S.T.; Alves, O.L. Nanocomposites based on graphene oxide and mesoporous silica nanoparticles: Preparation, characterization and nanobiointeractions with red blood cells and human plasma proteins. Appl. Surf. Sci. 2018, 437, 110–121. [Google Scholar] [CrossRef]
- Chetwynd, A.J.; Wheeler, K.E.; Lynch, I. Best practice in reporting corona studies: Minimum Information about Nanomaterial Biocorona Experiments (MINBE). Nano Today 2019, 28, 100758. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, S.; Yuan, B.; Li, Y.; Deng, Z. Toward a universal “adhesive nanosheet” for the assembly of multiple nanoparticles based on a protein-induced reduction/decoration of graphene oxide. J. Am. Chem. Soc. 2010, 132, 7279–7281. [Google Scholar] [CrossRef]
- Yang, P.; Liu, Q.; Liu, J.; Zhang, H.; Li, Z.; Li, R.; Liu, L.; Wang, J. Bovine serum albumin-coated graphene oxide for effective adsorption of uranium(VI) from aqueous solutions. Ind. Eng. Chem. Res. 2017, 56, 3588–3598. [Google Scholar] [CrossRef]
- Yu, X.; Sun, S.; Zhou, L.; Miao, Z.; Zhang, X.; Su, Z.; Wei, G. Removing metal ions from water with graphene–bovine serum albumin hybrid membrane. Nanomaterials 2019, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Zhang, Y.; Chen, W.; Wang, K.; Zhu, L. Concentration dependent effects of bovine serum albumin on graphene oxide colloidal stability in aquatic environment. Environ. Sci. Technol. 2018, 52, 7212–7219. [Google Scholar] [CrossRef]
- Shakiba, S.; Hakimian, A.; Barco, L.R.; Louie, S.M. Dynamic intermolecular interactions control adsorption from mixtures of natural organic matter and protein onto titanium dioxide nanoparticles. Environ. Sci. Technol. 2018, 52, 14158–14168. [Google Scholar] [CrossRef]
- Liu, X.; Yan, C.; Chen, K.L. Adsorption of human serum albumin on graphene oxide: Implications for protein corona formation and conformation. Environ. Sci. Technol. 2018, 53, 8631–8639. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Cheng, T.; Shang, J. Effect of bovine serum albumin on stability and transport of kaolinite colloid. Water Res. 2019, 155, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, Y.; Liu, Q.; Yan, C.; Xiao, B.; Yang, J.; Liu, M.; Zhu, L. Lateral size dependent colloidal stability of graphene oxide in water: Impacts of protein properties and water chemistry. Environ. Sci. Nano 2020, 7, 634–644. [Google Scholar] [CrossRef]
- Deng, R.; Lin, D.; Zhu, L.; Majumdar, S.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk. Nanotoxicology 2017, 11, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.L.M.; Lynch, I.; Peijnenburg, W.J.G.M.; Rumble, J.; Klaessig, F.; Marquardt, C.; Rauscher, H.; Puzyn, T.; Purian, R.; Åberg, C.; et al. How should the completeness and quality of curated nanomaterial data be evaluated? Nanoscale 2016, 8, 9919–9943. [Google Scholar] [CrossRef]
- Barnard, A.S.; Motevalli, B.; Parker, A.J.; Fischer, J.M.; Feigl, C.A.; Opletal, G.; Fischer, M. Nanoinformatics, and the big challenges for the science of small things. Nanoscale 2019, 11, 19190–19201. [Google Scholar] [CrossRef]
- Brown, K.A.; Brittman, S.; Maccaferri, N.; Jariwala, D.; Celano, U. Machine learning in nanoscience: Big data at small scales. Nano Lett. 2019, 20, 2–10. [Google Scholar] [CrossRef]
- Afantitis, A.; Melagraki, G.; Isigonis, P.; Tsoumanis, A.; Varsou, D.D.; Valsami-Jones, E.; Papadiamantis, A.; Ellis, L.-J.A.; Sarimveis, H.; Doganis, P.; et al. NanoSolveIT Project: Driving nanoinformatics research to develop innovative and integrated tools for in silico nanosafety assessment. Comput. Struct. Biotechnol. J. 2020, 18, 583–602. [Google Scholar] [CrossRef]
- Labouta, H.I.; Asgarian, N.; Rinker, K.D.; Cramb, D.T. Meta-analysis of nanoparticle cytotoxicity via data-mining the literature. ACS Nano 2019, 13, 1583–1594. [Google Scholar] [CrossRef]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef]
- Bilal, M.; Oh, E.; Liu, R.; Breger, J.C.; Medintz, I.L.; Cohen, Y. Bayesian network resource for meta-analysis: Cellular toxicity of quantum dots. Small 2019, 15, e1900510. [Google Scholar] [CrossRef] [PubMed]
- Geitner, N.K.; Hendren, C.O.; Cornelis, G.; Kaegi, R.; Lead, J.R.; Lowry, G.V.; Lynch, I.; Nowack, B.; Petersen, E.J.; Bernhardt, E.; et al. Harmonizing across environmental nanomaterial testing media for increased comparability of nanomaterial datasets. Environ. Sci. Nano 2020, 7, 13–36. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, J.M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; Santos, L.O.B.D.S.; Bourne, P.E.; et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kortenkamp, A.; Faust, M. Regulate to reduce chemical mixture risk. Science 2018, 361, 224–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escher, B.I.; Stapleton, H.M.; Schymanski, E.L. Tracking complex mixtures of chemicals in our changing environment. Science 2020, 367, 388–392. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Wang, J.; Wang, J.; Wang, X.; Chen, S.; Zhao, G.; Wu, L.; Xu, A. Mechanisms involved in the impact of engineered nanomaterials on the joint toxicity with environmental pollutants. Ecotoxicol. Environ. Saf. 2018, 162, 92–102. [Google Scholar] [CrossRef]
- Naasz, S.; Altenburger, R.; Kühnel, D. Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Sci. Total. Environ. 2018, 635, 1170–1181. [Google Scholar] [CrossRef]
- De Medeiros, A.M.Z.; Côa, F.; Alves, O.L.; Martinez, D.S.T.; Barbieri, E. Metabolic effects in the freshwater fish Geophagus iporangensis in response to single and combined exposure to graphene oxide and trace elements. Chemosphere 2020, 243, 125316. [Google Scholar] [CrossRef]
- Kim, K.T.; Klaine, S.J.; Lin, S.; Ke, P.C.; Kim, S.D. Acute toxicity of a mixture of copper and single-walled carbon nanotubes toDaphnia magna. Environ. Toxicol. Chem. 2010, 29, 122–126. [Google Scholar] [CrossRef]
- Revel, M.; Fournier, M.; Robidoux, P.Y. Toxic effect of single walled carbon nanotubes combined with cadmium to the crustacean daphnia magna. Int. Lett. Nat. Sci. 2015, 42, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Ni, L.; Li, Y. Role of graphene oxide in mitigated toxicity of heavy metal ions on Daphnia magna. RSC Adv. 2018, 8, 41358–41367. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Fan, W.; Xu, Z.; Peng, W.; Luo, S. Comparative effects of graphene and graphene oxide on copper toxicity to Daphnia magna: Role of surface oxygenic functional groups. Environ. Pollut. 2018, 236, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Nasser, F.; Lynch, I. Updating traditional regulatory tests for use with novel materials: Nanomaterial toxicity testing with Daphnia magna. Saf. Sci. 2019, 118, 497–504. [Google Scholar] [CrossRef]
- Ellis, L.-J.A.; Valsami-Jones, E.; Lynch, I. Exposure medium and particle ageing moderate the toxicological effects of nanomaterials to Daphnia magna over multiple generations: A case for standard test review? Environ. Sci. Nano 2020, 7, 1136–1149. [Google Scholar] [CrossRef] [Green Version]
- Lucidchart. Online Diagram Software and Visual Solution. Available online: https://www.lucidchart.com/pages/ (accessed on 15 May 2020).
- SciNote. Free Electronic Lab Notebook (ELN). Available online: https://www.scinote.net/ (accessed on 15 May 2020).
- Hastings, J.; Jeliazkova, N.; Owen, G.; Tsiliki, G.; Munteanu, C.R.; Steinbeck, C.; Willighagen, E. eNanoMapper: Harnessing ontologies to enable data integration for nanomaterial risk assessment. J. Biomed. Semant. 2015, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 1998, 33, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Robinson, R.L.M.; Cronin, M.T.D.; Richarz, A.-N.; Rallo, R. An ISA-TAB-Nano based data collection framework to support data-driven modelling of nanotoxicology. Beilstein J. Nanotechnol. 2015, 6, 1978–1999. [Google Scholar] [CrossRef]
- Sun, B.; Fernandez, M.; Barnard, A.S. Statistics, damned statistics and nanoscience—Using data science to meet the challenge of nanomaterial complexity. Nanoscale Horiz. 2016, 1, 89–95. [Google Scholar] [CrossRef]
- Izak-Nau, E.; Huk, A.; Reidy, B.; Uggerud, H.; Vadset, M.; Eiden, S.; Voetz, M.; Himly, M.; Duschl, A.; Dusinska, M.; et al. Impact of storage conditions and storage time on silver nanoparticles’ physicochemical properties and implications for their biological effects. RSC Adv. 2015, 5, 84172–84185. [Google Scholar] [CrossRef] [Green Version]
- Mitrano, D.M.; Motellier, S.; Clavaguera, S.; Nowack, B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ. Int. 2015, 77, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Aksay, I.A. Factors controlling the size of graphene oxide sheets produced via the graphite oxide route. ACS Nano 2011, 5, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, M.; Martins, C.H.Z.; Franqui, L.S.; Fonseca, L.C.; Delite, F.S.; Lanzoni, E.M.; Martinez, D.S.T.; Alves, O.L. Covalent functionalization of graphene oxide with d-mannose: Evaluating the hemolytic effect and protein corona formation. J. Mater. Chem. B 2018, 6, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- Clemente, Z.; Silva, G.H.; Nunes, M.C.D.S.; Martinez, D.S.T.; Maurer-Morelli, C.V.; Thomaz, A.A.; Castro, V.L. Exploring the mechanisms of graphene oxide behavioral and morphological changes in zebrafish. Environ. Sci. Pollut. Res. 2019, 26, 30508–30523. [Google Scholar] [CrossRef]
- Clemente, Z.; Castro, V.L.; Franqui, L.S.; Silva, C.A.; Martinez, D.S.T. Nanotoxicity of graphene oxide: Assessing the influence of oxidation debris in the presence of humic acid. Environ. Pollut. 2017, 225, 118–128. [Google Scholar] [CrossRef]
- Franqui, L.S.; De Farias, M.A.; Portugal, R.V.; Costa, C.A.; Domingues, R.R.; Filho, A.G.S.; Coluci, V.R.; Leme, A.F.P.; Martinez, D.S.T. Interaction of graphene oxide with cell culture medium: Evaluating the fetal bovine serum protein corona formation towards in vitro nanotoxicity assessment and nanobiointeractions. Mater. Sci. Eng. C 2019, 100, 363–377. [Google Scholar] [CrossRef]
- Emadi, F.; Amini, A.; Gholami, A.; Ghasemi, Y. Functionalized graphene oxide with chitosan for protein nanocarriers to protect against enzymatic cleavage and retain collagenase activity. Sci. Rep. 2017, 7, 42258. [Google Scholar] [CrossRef] [Green Version]
- Mudunkotuwa, I.A.; Grassian, V.H. Biological and environmental media control oxide nanoparticle surface composition: The roles of biological components (proteins and amino acids), inorganic oxyanions and humic acid. Environ. Sci. Nano 2015, 2, 429–439. [Google Scholar] [CrossRef]
- Jiang, Y.; Raliya, R.; Fortner, J.D.; Biswas, P. Graphene oxides in water: Correlating morphology and surface chemistry with aggregation behavior. Environ. Sci. Technol. 2016, 50, 6964–6973. [Google Scholar] [CrossRef]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 333–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Zhang, W.; Yu, X.; Wang, Z.; Su, Z.; Wei, G. When biomolecules meet graphene: From molecular level interactions to material design and applications. Nanoscale 2016, 8, 19491–19509. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Bian, Z.; Zhang, J.-X.; Ding, A.-Z.; Liu, S.-L.; Wang, H. Effect of the oxygen-containing functional group of graphene oxide on the aqueous cadmium ions removal. Appl. Surf. Sci. 2015, 329, 269–275. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Wang, X.; Ma, Y.; Yang, Y.; Zhuang, L.; Zhang, S.; Jehan, R.; Chen, J.; Wang, X. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: A review. Environ. Pollut. 2019, 252, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, M.; Jiang, L.X.; Song, L. New insight into molecular interaction of heavy metal pollutant—Cadmium (II) with human serum albumin. Environ. Sci. Pollut. Res. 2014, 21, 6994–7005. [Google Scholar] [CrossRef]
- Seredych, M.; Mikhalovska, L.; Mikhalovsky, S.; Gogotsi, Y. Adsorption of bovine serum albumin on carbon-based materials. C J. Carbon Res. 2018, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Sanchís, J.; Olmos, M.; Vincent, P.; Farré, M.; Barceló, D. New insights on the influence of organic co-contaminants on the aquatic toxicology of carbon nanomaterials. Environ. Sci. Technol. 2015, 50, 961–969. [Google Scholar] [CrossRef]
- Montagner, A.; Bosi, S.; Tenori, E.; Bidussi, M.; Alshatwi, A.A.; Tretiach, M.; Prato, M.; Syrgiannis, Z. Ecotoxicological effects of graphene-based materials. 2D Mater. 2016, 4, 012001. [Google Scholar] [CrossRef]
- Lv, X.; Yang, Y.; Tao, Y.; Jiang, Y.; Chen, B.; Zhu, X.; Cai, Z.; Li, B. A mechanism study on toxicity of graphene oxide to Daphnia magna: Direct link between bioaccumulation and oxidative stress. Environ. Pollut. 2018, 234, 953–959. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; White, J.C.; Xing, B. Graphene in the aquatic environment: Adsorption, dispersion, toxicity and transformation. Environ. Sci. Technol. 2014, 48, 9995–10009. [Google Scholar] [CrossRef]
- Ren, X.; Li, J.; Chen, C.; Gao, Y.; Chen, D.; Su, M.; Alsaedi, A.; Hayat, T. Graphene analogues in aquatic environments and porous media: Dispersion, aggregation, deposition and transformation. Environ. Sci. Nano 2018, 5, 1298–1340. [Google Scholar] [CrossRef]
- Castro, V.L.; Clemente, Z.; Jonsson, C.; Silva, M.; Vallim, J.H.; De Medeiros, A.M.Z.; Martinez, D.S.T. Nanoecotoxicity assessment of graphene oxide and its relationship with humic acid. Environ. Toxicol. Chem. 2018, 37, 1998–2012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, T.; Shi, L.; Guo, X.; Si, X.; Yang, R.; Quan, X. The effects of humic acid on the toxicity of graphene oxide to Scenedesmus obliquus and Daphnia magna. Sci. Total. Environ. 2019, 649, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total. Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Yang, J.H.; Kim, H.J.; Lee, S.M.; Kim, B.-M.; Seo, Y.R. Cadmium-induced biomarkers discovery and comparative network analysis in Daphnia magna. Mol. Cell. Toxicol. 2017, 13, 327–336. [Google Scholar] [CrossRef]
- Fan, W.-H.; Tang, G.; Zhao, C.-M.; Duan, Y.; Zhang, R. Metal accumulation and biomarker responses in daphnia magna following cadmium and zinc exposure. Environ. Toxicol. Chem. 2009, 28, 305–310. [Google Scholar] [CrossRef]
- Qu, R.-J.; Wang, X.-H.; Feng, M.-B.; Li, Y.; Liu, H.-X.; Wang, L.-S.; Wang, Z.-Y. The toxicity of cadmium to three aquatic organisms (Photobacterium phosphoreum, Daphnia magna and Carassius auratus) under different pH levels. Ecotoxicol. Environ. Saf. 2013, 95, 83–90. [Google Scholar] [CrossRef]
- Penttinen, S.; Kostamo, A.; Kukkonen, J.V.K. Combined effects of dissolved organic material and water hardness on toxicity of cadmium to Daphnia magna. Environ. Toxicol. Chem. 1998, 17, 2498–2503. [Google Scholar] [CrossRef]
- Lin, H.; Xia, X.; Jiang, X.; Bi, S.; Wang, H.; Zhai, Y.; Wen, W.; Guo, X. Bioavailability of pyrene associated with different types of protein compounds: Direct evidence for its uptake by Daphnia magna. Environ. Sci. Technol. 2018, 52, 9851–9860. [Google Scholar] [CrossRef]
- De Melo, C.B.; Côa, F.; Alves, O.L.; Martinez, D.S.T.; Barbieri, E. Co-exposure of graphene oxide with trace elements: Effects on acute ecotoxicity and routine metabolism in Palaemon pandaliformis (shrimp). Chemosphere 2019, 223, 157–164. [Google Scholar] [CrossRef]
- Markiewicz, M.; Kumirska, J.; Lynch, I.; Matzke, M.; Köser, J.; Bemowsky, S.; Docter, D.; Stauber, R.H.; Westmeier, D.; Stolte, S. Changing environments and biomolecule coronas: Consequences and challenges for the design of environmentally acceptable engineered nanoparticles. Green Chem. 2018, 20, 4133–4168. [Google Scholar] [CrossRef]
- Khan, L.U.; Petry, R.; Paula, A.J.; Knobel, M.; Martinez, D.S.T. Protein corona formation on magnetic nanoparticles conjugated with luminescent europium complexes. ChemNanoMat 2018, 4, 1202–1208. [Google Scholar] [CrossRef]
- Chetwynd, A.J.; Lynch, I. The rise of the nanomaterial metabolite corona, and emergence of the complete corona. Environ. Sci. Nano 2020, 7, 1041–1060. [Google Scholar] [CrossRef]
- Nasser, F.; Lynch, I. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J. Proteom. 2016, 137, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattsson, K.; Aguilar, R.; Torstensson, O.; Perry, D.; Bernfur, K.; Linse, S.; Hansson, L.-A.; Åkerfeldt, K.S.; Cedervall, T. Disaggregation of gold nanoparticles by Daphnia magna. Nanotoxicology 2018, 12, 885–900. [Google Scholar] [CrossRef] [Green Version]
- Briffa, S.M.; Nasser, F.; Valsami-Jones, E.; Lynch, I. Uptake and impacts of polyvinylpyrrolidone (PVP) capped metal oxide nanoparticles on Daphnia magna: Role of core composition and acquired corona. Environ. Sci. Nano 2018, 5, 1745–1756. [Google Scholar] [CrossRef] [Green Version]
- Grintzalis, K.; Lawson, T.N.; Nasser, F.; Lynch, I.; Viant, M.R. Metabolomic method to detect a metabolite corona on amino-functionalized polystyrene nanoparticles. Nanotoxicology 2019, 13, 783–794. [Google Scholar] [CrossRef]
- Martinez, D.S.T.; Paula, A.J.; Fonseca, L.C.; De Luna, L.A.; Silveira, C.P.; Durán, N.; Alves, O.L. Monitoring the hemolytic effect of mesoporous silica nanoparticles after human blood protein corona formation. Eur. J. Inorg. Chem. 2015, 2015, 4595–4602. [Google Scholar] [CrossRef]
- Nasser, F.; Constantinou, J.; Lynch, I. Nanomaterials in the environment acquire an “eco-corona” impacting their toxicity to daphnia magna—A call for updating toxicity testing policies. Proteomics 2019, 20, e1800412. [Google Scholar] [CrossRef] [Green Version]






| Materials | Ultrapure Water | Reconstituted Water | ||||
|---|---|---|---|---|---|---|
| HD (nm) | PdI | ZP (mV) | HD (nm) | PdI | ZP (mV) | |
| GO | 196.7 ± 2.8 | 0.232 ± 0.007 | − 31.9 ± 5.9 | 1490.3 ± 117.5 | 0.615 ± 0.060 | − 16.3 ± 0.6 |
| BSA@GO | 975.8 ± 123.1 | 0.834 ± 0.100 | − 35.8 ± 1.7 | 1715.0 ± 56.5 | 0.798 ± 0.031 | − 19.3 ± 1.2 |
| Treatments | EC50 (mg L−1) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Cd2+ | 0.35 (0.3 to 0.41) | 0.18 (0.15 to 0.21) | 0.11 (0.09 to 0.13) |
| Cd2+ + GO (0.1 mg L−1) | 0.64 (0.54 to 0.74) | 0.20 (0.17 to 0.23) | 0.12 (0.10 to 0.14) |
| Cd2+ + GO (1.0 mg L−1) | 0.79 (0.70 to 0.86) | 0.29 (0.20 to 0.42) | 0.16 (0.13 to 0.19) |
| Cd2+ + GO (10 mg L−1) | 1.0 (0.86 to 1.17) | 0.48 (0.42 to 0.55) | 0.20 (0.17 to 0.23) |
| Cd2+ + BSA@GO (0.1 mg L−1) | 0.71 (0.58 to 0.87) | 0.30 (0.25 to 0.37) | 0.10 (0.08 to 0.13) |
| Cd2+ + BSA@GO (1.0 mg L−1) | 0.95 (0.82 to 1.10) | 0.61 (0.53 to 0.70) | 0.21 (0.18 to 0.24) |
| Cd2+ + BSA@GO (10 mg L−1) | 1.43 (1.26 to 1.63) | 1.17 (1.04 to 1.33) | 0.76 (0.68 to 0.86) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, D.S.T.; Da Silva, G.H.; de Medeiros, A.M.Z.; Khan, L.U.; Papadiamantis, A.G.; Lynch, I. Effect of the Albumin Corona on the Toxicity of Combined Graphene Oxide and Cadmium to Daphnia magna and Integration of the Datasets into the NanoCommons Knowledge Base. Nanomaterials 2020, 10, 1936. https://doi.org/10.3390/nano10101936
Martinez DST, Da Silva GH, de Medeiros AMZ, Khan LU, Papadiamantis AG, Lynch I. Effect of the Albumin Corona on the Toxicity of Combined Graphene Oxide and Cadmium to Daphnia magna and Integration of the Datasets into the NanoCommons Knowledge Base. Nanomaterials. 2020; 10(10):1936. https://doi.org/10.3390/nano10101936
Chicago/Turabian StyleMartinez, Diego Stéfani T., Gabriela H. Da Silva, Aline Maria Z. de Medeiros, Latif U. Khan, Anastasios G. Papadiamantis, and Iseult Lynch. 2020. "Effect of the Albumin Corona on the Toxicity of Combined Graphene Oxide and Cadmium to Daphnia magna and Integration of the Datasets into the NanoCommons Knowledge Base" Nanomaterials 10, no. 10: 1936. https://doi.org/10.3390/nano10101936