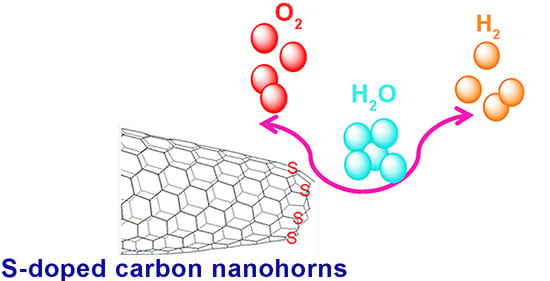
Sulfur-Doped Carbon Nanohorn Bifunctional Electrocatalyst for Water Splitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ox-CNHs
2.2. Preparation of S-Doped CNHs
2.3. General
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, J.; Wie, C.; Huang, Z.-F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.-J.; Wang, Z.L. Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.L.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater. 2019, 31, 1806296. [Google Scholar] [CrossRef] [PubMed]
- Gloag, L.; Benedetti, T.M.; Cheong, S.; Li, Y.; Chan, X.-H.; Lacroix, L.-M.; Chang, S.L.Y.; Arenal, R.; Florea, I.; Barron, H.; et al. Three-dimensional branched and faceted gold–ruthenium nanoparticles: Using nanostructure to improve stability in oxygen evolution electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 10241–10245. [Google Scholar] [CrossRef] [PubMed]
- Kagkoura, A.; Canton-Vitoria, R.; Vallan, L.; Hernandez-Ferrer, J.; Benito, A.M.; Maser, W.K.; Arenal, R.; Tagmatarchis, N. Bottom-up synthesized MoS2 interfacing polymer carbon nanodots with electrocatalytic activity for hydrogen evolution. Chem. Eur. J. 2020, 26, 6635–6642. [Google Scholar] [CrossRef]
- Kagkoura, A.; Tzanidis, I.; Dracopoulos, V.; Tagmatarchis, N.; Tasis, D. Template synthesis of defect-rich MoS2-based assemblies as electrocatalytic platforms for hydrogen evolution reaction. Chem. Commun. 2019, 55, 2078–2081. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liao, X.; Pan, X.; Yan, M.; Li, Y.; Tian, X.; Zhao, Y.; Xu, L.; Mai, L. Superior hydrogen evolution reaction performance in 2H-MoS2 to that of 1T phase. Small 2019, 15, 1900964. [Google Scholar] [CrossRef]
- Jia, X.; Ren, H.; Hu, H.; Song, Y.-F. 3D Carbon foam supported edge-rich N-doped MoS2 nanoflakes for enhanced electrocatalytic hydrogen evolution. Chem. Eur. J. 2020, 26, 4150–4156. [Google Scholar] [CrossRef]
- Yi, X.; He, X.; Yin, F.; Chen, B.; Li, G.; Yin, H. One-step synthesis of oxygen incorporated V–MoS2 supported on partially sulfurized nickel foam as a highly active catalyst for hydrogen evolution. Int. J. Hydrogen Energy 2020, 45, 2774–2784. [Google Scholar] [CrossRef]
- Bolar, S.; Shit, S.; Kumar, J.S.; Murmu, N.C.; Ganesh, R.S.; Inokawa, H.; Kuila, T. Optimization of active surface area of flower like MoS2 using V-doping towards enhanced hydrogen evolution reaction in acidic and basic medium. Appl. Catal. B 2019, 254, 432–442. [Google Scholar] [CrossRef]
- Mohanty, B.; Ghorbani-Asl, M.; Kretschmer, S.; Ghosh, A.; Guha, P.; Panda, S.K.; Jena, B.; Krasheninnikov, A.V.; Jena, B.K. MoS2 Quantum dots as efficient catalyst materials for the oxygen evolution reaction. ACS Catal. 2018, 8, 1683–1689. [Google Scholar] [CrossRef]
- Badruzzaman, A.; Yuda, A.; Ashock, A.; Kumar, A. Recent advances in cobalt based heterogeneous catalysts for oxygen evolution reaction. Inorg. Chim. Acta 2020, 511, 119854. [Google Scholar] [CrossRef]
- Tang, B.; Yu, Z.G.; Seng, H.L.; Zhang, N.; Liu, X.; Zhang, Y.-W.; Yang, W.; Gong, H. Simultaneous edge and electronic control of MoS2 nanosheets through Fe doping for an efficient oxygen evolution reaction. Nanoscale 2018, 10, 20113–20119. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tu, W.; Zhang, B.; Yin, S.; Huang, Y.; Kraft, M.; Xu, R. Nickel nanoparticles encapsulated in few-layer nitrogen-doped graphene derived from metal–organic frameworks as efficient bifunctional electrocatalysts for overall water splitting. Adv. Mater. 2017, 29, 1605957. [Google Scholar] [CrossRef]
- Kagkoura, A.; Tagmatarchis, N. Carbon nanohorn-based electrocatalysts for energy conversion. Nanomaterials 2020, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, properties, functionalization, and applications of carbon nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef]
- Pippa, N.; Stangel, C.; Kastanas, I.; Triantafyllopoulou, E.; Naziris, N.; Stellas, D.; Zhang, M.; Yudasaka, M.; Demetzos, C.; Tagmatarchis, N. Carbon nanohorn/liposome systems: Preformulation, design and in vitro toxicity studies. Mater. Sci. Eng. C 2019, 105, 110114. [Google Scholar] [CrossRef]
- Stergiou, A.; Perivoliotis, D.K.; Tagmatarchis, N. (Photo)electrocatalysis of molecular oxygen reduction by S-doped graphene decorated with a star-shaped oligothiophene. Nanoscale 2019, 11, 7335–7346. [Google Scholar] [CrossRef]
- Dumont, J.H.; Martinez, U.; Artyushkova, K.; Purdy, G.M.; Dattelbaum, A.M.; Zelenay, P.; Mohite, A.; Atanassov, P.; Gupta, G. Nitrogen-doped graphene oxide electrocatalysts for the oxygen reduction reaction. ACS Appl. Nano Mater. 2019, 2, 1675–1682. [Google Scholar] [CrossRef]
- Kagkoura, A.; Pelaez-Fernandez, M.; Arenal, R.; Tagmatarchis, N. Sulfur-doped graphene/transition metal dichalcogenide heterostructured hybrids with electrocatalytic activity toward the hydrogen evolution reaction. Nanoscale Adv. 2019, 1, 1489–1496. [Google Scholar] [CrossRef] [Green Version]
- Perivoliotis, D.K.; Sato, Y.; Suenaga, K.; Tagmatarchis, N. Sulfur-doped graphene-supported nickel-core palladium-shell nanoparticles as efficient oxygen reduction and methanol oxidation electrocatalyst. ACS Appl. Energy Mater. 2018, 1, 3869–3880. [Google Scholar] [CrossRef]
- Wu, X.; Cui, L.; Tang, P.; Hu, Z.; Ma, D.; Shi, Z. Synthesis and catalytic activity of heteroatom doped metal-free single-wall carbon nanohorns. Chem. Commun. 2016, 52, 5391–5393. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.H.; Kim, C.H.; Lee, H.S.; Choi, G.B.; Kim, D.W.; Yang, C.M.; Kim, Y.A. Enriched pyridinic nitrogen atoms at nanoholes of carbon nanohorns for efficient oxygen reduction. Sci. Rep. 2019, 9, 20170. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, L.; Tang, P.; Li, M.; Shi, Z. Influence of nitrogen precursors on the structure, composition, and oxygen reduction reaction performance of dual heteroatom doped carbon nanohorns. RSC Adv. 2016, 6, 63730–63735. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, J.; Wu, X.; Wang, Y.; Li, M.; Shi, Z. Palladium nanoparticles loaded on nitrogen and boron dual-doped single-wall carbon nanohorns with high electrocatalytic activity in the oxygen reduction reaction. RSC Adv. 2018, 8, 33688–33694. [Google Scholar] [CrossRef] [Green Version]
- Maciasa, E.M.; Valenzuela-Muñiz, A.M.; Alonso-Núñez, G.; Farías Sánchez, M.H.; Gauvinc, R.; Gómez, Y.V. Sulfur doped carbon nanohorns towards oxygen reduction reaction. Diam. Relat. Mater. 2020, 103, 107671. [Google Scholar] [CrossRef]
- Unni, S.M.; Bhange, S.N.; Illathvalappil, R.; Mutneja, N.; Patil, K.R.; Kurungot, R. Nitrogen-induced surface area and conductivity modulation of carbon nanohorn and its function as an efficient metal-free oxygen reduction electrocatalyst for anion-exchange membrane fuel cells. Small 2015, 11, 352–360. [Google Scholar] [CrossRef]
- Devadas, B.; Imae, T. Hydrogen evolution reaction efficiency by low loading of platinum nanoparticles protected by dendrimers on carbon materials. Electrochem. Commun. 2016, 72, 135–139. [Google Scholar] [CrossRef]
- Devadas, B.; Chang, C.C.; Imae, T. Hydrogen evolution reaction efficiency of carbon nanohorn incorporating molybdenum sulfide and polydopamine/palladium nanoparticles. Taiwan Inst. Chem. Eng. 2019, 102, 378–386. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, Y.; Nan, Y.; Li, B.; Song, X. Ni-Based nanoparticle-embedded N-doped carbon nanohorns derived from double core–shell CNH@PDA@NiMOFs for oxygen electrocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 12743–12754. [Google Scholar] [CrossRef]
- Yang, S.; Zhi, L.; Tang, K.; Feng, X.; Maier, J.; Müllen, K. Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reactions. Adv. Funct. Mater. 2012, 22, 3634–3640. [Google Scholar] [CrossRef]
- Herrmann, I.; Kramm, U.I.; Radnik, J.S.; Fiechter, S.; Bogdanoff, P. Influence of sulfur on the pyrolysis of CoTMPP as electrocatalyst for the oxygen reduction reaction. J. Electrochem. 2009, 156, B1283–B1292. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, K.; Jiang, B.; Li, J.; Zeng, M.; Fu, L. Emerging two-dimensional nanomaterials for electrochemical hydrogen evolution. J. Mater. Chem. A 2017, 5, 8187–8208. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, C.; Xie, Y.; Tumlin, T.; Giri, L.; Karna, S.P.; Lin, J. Laser induced MoS2/carbon hybrids for hydrogen evolution reaction catalysts. J. Mater. Chem. A 2016, 4, 6824–6830. [Google Scholar] [CrossRef] [Green Version]
- You, B.; Jiang, N.; Sheng, M.; Bhushan, M.W.; Sun, Y. Hierarchically Porous Urchin-like Ni2P Superstructures Supported on Nickel Foam as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. ACS Catal. 2016, 6, 714–721. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef] [Green Version]







| Catalyst | Potential (V vs. RHE) at 10 mA/cm2 | Overpotential (mV vs. RHE) at 10 mA/cm2 | Tafel Slope (mV/dec) | ECSA (cm2) b | Rct (Ω) |
|---|---|---|---|---|---|
| S-CNHs | 1.63 | 400 | 80 | 67.5 | 10.8 |
| S-CNHs a | 1.65 | 420 | 84 | 61.25 | - |
| ox-CNHs | 1.82 | 590 | 202 | 60 | 560.5 |
| ox-CNHs a | 2.5 | - | 279 | 13.75 | - |
| pristine CNHs | 1.82 | 590 | 333 | 19.4 | 450.5 |
| pristine CNHs a | 2.5 | - | 310 | 4.7 | - |
| Catalyst | Onset Potential (V vs. RHE) | Potential (V vs. RHE) at −10 mA/cm2 | Tafel Slope (mV/dec) | ECSA (cm2) b | Rct (Ω) |
|---|---|---|---|---|---|
| S-CNHs | −0.2 | −0.29 | 82 | 205 | 30.4 |
| S-CNHs a | −0.21 | −0.33 | 82 | 190 | - |
| ox-CNHs | −0.25 | −0.43 | 240 | 130 | 197 |
| ox-CNHs a | −0.26 | −0.48 | 245 | 102 | - |
| pristine CNHs | −0.34 | −0.75 | 320 | 47.5 | 123.4 |
| pristine CNHs a | −0.35 | −0.79 | 320 | 31.25 | - |
| Pt/C | 0.034 | −0.0025 | 35 | 364 | 6.1 |
| Pt/C a | 0.011 | −0.02 | 35 | 350 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kagkoura, A.; Arenal, R.; Tagmatarchis, N. Sulfur-Doped Carbon Nanohorn Bifunctional Electrocatalyst for Water Splitting. Nanomaterials 2020, 10, 2416. https://doi.org/10.3390/nano10122416
Kagkoura A, Arenal R, Tagmatarchis N. Sulfur-Doped Carbon Nanohorn Bifunctional Electrocatalyst for Water Splitting. Nanomaterials. 2020; 10(12):2416. https://doi.org/10.3390/nano10122416
Chicago/Turabian StyleKagkoura, Antonia, Raul Arenal, and Nikos Tagmatarchis. 2020. "Sulfur-Doped Carbon Nanohorn Bifunctional Electrocatalyst for Water Splitting" Nanomaterials 10, no. 12: 2416. https://doi.org/10.3390/nano10122416