Reduction in Processing Time in Ca3Co4O9+δ Ceramics through Nanoprecursors Produced by an Easily Scalable and Environmentally Friendly Process
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
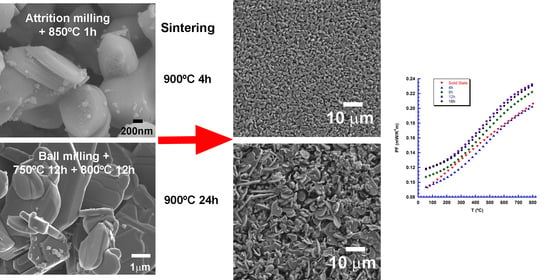
3.1. Precursor Powders
3.2. Sintered Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rowe, D.M. Thermoelectrics Handbook: Macro to Nano, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1-3–1-7. [Google Scholar] [CrossRef]
- Santamaria, J.M.; Alkorta, J.; Sevillano, J.G. Mechanical properties of bismuth telluride (Bi2Te3) processed by high pressure torsion (HPT). Bol. Soc. Esp. Ceram. V. 2013, 52, 137–142. [Google Scholar] [CrossRef]
- Terasaki, I.; Sasago, Y.; Uchinokura, K. Large thermoelectric power in NaCo2O4 single crystals. Phys. Rev. B 1997, 56, 12685–12687. [Google Scholar] [CrossRef]
- Yaroshevsky, A.A. Abundances of chemical elements in the Earth’s crust. Geochem. Int. 2006, 44, 48–55. [Google Scholar] [CrossRef]
- Sotelo, A.; Rasekh, S.; Constantinescu, G.; Torres, M.A.; Madre, M.A.; Diez, J.C. Improvement of textured Bi1.6Pb0.4Sr2Co1.8Ox thermoelectric performances by metallic Ag additions. Ceram. Int. 2013, 39, 1597–1602. [Google Scholar] [CrossRef] [Green Version]
- Masset, A.C.; Michel, C.; Maignan, A.; Hervieu, M.; Toulemonde, O.; Studer, F.; Raveau, B.; Hejtmanek, J. Misfit-layered cobaltite with an anisotropic giant magnetoresistance: Ca3Co4O9. Phys. Rev. B 2000, 62, 166–175. [Google Scholar] [CrossRef]
- Delorme, F.; Ovono Ovono, D.; Marudhachalam, P.; Fernandez Martin, C.; Fraboulet, O. Effect of precursors size on the thermoelectric properties of Ca3Co4O9 ceramics. Mater. Res. Bull. 2012, 47, 1169–1175. [Google Scholar] [CrossRef]
- Sotelo, A.; Costa, F.M.; Ferreira, N.M.; Kovalevsky, A.; Ferro, M.C.; Amaral, V.S.; Amaral, J.S.; Rasekh, S.; Torres, M.A.; Madre, M.A.; et al. Tailoring Ca3Co4O9 microstructure and performances using a transient liquid phase sintering additive. J. Eur. Ceram. Soc. 2016, 36, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Delorme, F.; Diaz-Chao, P.; Giovannelli, F. Effect of Ca substitution by Fe on the thermoelectric properties of Ca3Co4O9 ceramics. J. Electroceram. 2018, 40, 107–114. [Google Scholar] [CrossRef]
- Diez, J.C.; Torres, M.A.; Rasekh, S.; Constantinescu, G.; Madre, M.A.; Sotelo, A. Enhancement of Ca3Co4O9 thermoelectric properties by Cr for Co substitution. Ceram. Int. 2013, 39, 6051–6056. [Google Scholar] [CrossRef] [Green Version]
- Woermann, E.; Muan, A. Phase equilibria in the system CaO-cobalt oxide in air. J. Inorg. Nucl. Chem. 1970, 32, 1455–1459. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Muguerra, H.; Traianidis, M.; Henrist, C.; Vertruyen, B.; Cloots, R. Enhancement of the power factor of [Bi1.68Ca2O4]RS[CoO2]1.69—Ag composites prepared by the spray-drying method. Solid State Sci. 2010, 12, 1490–1495. [Google Scholar] [CrossRef]
- Delorme, F.; Diaz-Chao, P.; Guilmeau, E.; Giovannelli, F. Thermoelectric properties of Ca3Co4O9–Co3O4 composites. Ceram. Int. 2015, 41, 10038–10043. [Google Scholar] [CrossRef]
- Kenfaui, D.; Gomina, M.; Noudem, J.G.; Chateigner, D. Anisotropy of transport properties correlated to grain boundary density and quantified texture in thick oriented Ca3Co4O9 ceramics. Materials 2018, 11, 1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klyndyuk, A.I.; Matsukevich, I.V.; Janek, M.; Chizhova, E.A.; Lences, Z.; Hanzel, O.; Veteska, P. Thermoelectric properties of a phase-heterogeneous ceramic based on Ca3Co4O9+δ, prepared by hot pressing. Russ. J. Appl. Chem. 2020, 93, 1126–1131. [Google Scholar] [CrossRef]
- Torres, M.A.; Garcia, G.; Urrutibeascoa, I.; Madre, M.A.; Diez, J.C.; Sotelo, A. Fast preparation route to high-performances textured Sr-doped Ca3Co4O9 thermoelectric materials through precursor powder modification. Sci. China Mater. 2019, 62, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Schulz, T.; Topfer, J. Thermoelectric properties of Ca3Co4O9 ceramics prepared by an alternative pressure-less sintering/annealing method. J. Alloys Compd. 2016, 659, 122–126. [Google Scholar] [CrossRef]
- Klyndyuk, A.; Chizhova, E.; Matsukevich, I.; Tugova, E. Thermoelectric properties of inhomogeneous ceramics based on the layered calcium cobaltate. Univ. J. Mater. Sci. 2019, 7, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Sedmidubsky, D.; Jakes, V.; Jankovsky, O.; Leitner, J.; Sofer, Z.; Hejtmanek, J. Phase equilibria in Ca–Co–O system. J. Solid State Chem. 2012, 194, 199–205. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Matsukevich, I.V. Synthesis, structure, and properties of Ca3Co3.85M0.15O9+δ (M = Ti–Zn, Mo, W, Pb, Bi) layered thermoelectrics. Inorg. Mater. 2015, 51, 944–950. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Lan, J.; Shen, Z.; Liu, Y.; Nan, C.-W.; Li, J.-F. High-temperature electrical transport behaviors in textured Ca3Co4O9-based polycrystalline ceramics. Appl. Phys. Lett. 2009, 94, 072107. [Google Scholar] [CrossRef]
- Constantinescu, G.; Sarabando, A.R.; Rasekh, S.h.; Lopes, D.; Sergiienko, S.; Amirkhizi, P.; Frade, J.R.; Kovalevsky, A.V. Redox-promoted tailoring of the high-temperature electrical performance in Ca3Co4O9 thermoelectric materials by metallic cobalt addition. Materials 2020, 13, 1060. [Google Scholar] [CrossRef] [Green Version]
- Emerenciano, A.A.; Araujo, A.J.M.; Grilo, J.P.F.; Macedo, D.A.; Rasekh, S.; Kovalevsky, A.V.; Paskocimas, C.A.; Nascimento, R.M. Environmentally friendly synthesis methods to obtain the misfit [Ca2CoO3-δ]0.62[CoO2] thermoelectric material. Mater. Lett. 2019, 254, 286–289. [Google Scholar] [CrossRef]
- Sotelo, A.; Constantinescu, G.; Rasekh, S.; Torres, M.A.; Diez, J.C.; Madre, M.A. Improvement of thermoelectric properties of Ca3Co4O9 using soft chemistry synthetic methods. J. Eur. Ceram. Soc. 2012, 32, 2415–2422. [Google Scholar] [CrossRef] [Green Version]
- Madre, M.A.; Costa, F.M.; Ferreira, N.M.; Sotelo, A.; Torres, M.A.; Constantinescu, G.; Rasekh, S.; Diez, J.C. Preparation of high-performance Ca3Co4O9 thermoelectric ceramics produced by a new two-step method. J. Eur. Ceram. Soc. 2013, 33, 1747–1754. [Google Scholar] [CrossRef] [Green Version]
- Kenfaui, D.; Lenoir, B.; Chateigner, D.; Ouladdiaf, B.; Gomina, M.; Noudem, J.G. Development of multilayer textured Ca3Co4O9 materials for thermoelectric generators: Influence of the anisotropy on the transport properties. J. Eur. Ceram. Soc. 2012, 32, 2405–2414. [Google Scholar] [CrossRef]
- Wu, N.Y.; Holgate, T.C.; Nong, N.V.; Pryds, N.; Linderoth, S. High temperature thermoelectric properties of Ca3Co4O9+δ by auto-combustion synthesis and spark plasma sintering. J. Eur. Ceram. Soc. 2014, 34, 925–931. [Google Scholar] [CrossRef]
- Noudem, J.G.; Kenfaui, D.; Chateigner, D.; Gomina, M. Toward the enhancement of thermoelectric properties of lamellar Ca3Co4O9 by edge-free spark plasma texturing. Scr. Mater. 2012, 66, 258–260. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaveda, H.; Madre, M.A.; Mora, M.; Torres, M.A.; Sotelo, A. Reduction in Processing Time in Ca3Co4O9+δ Ceramics through Nanoprecursors Produced by an Easily Scalable and Environmentally Friendly Process. Nanomaterials 2020, 10, 2533. https://doi.org/10.3390/nano10122533
Amaveda H, Madre MA, Mora M, Torres MA, Sotelo A. Reduction in Processing Time in Ca3Co4O9+δ Ceramics through Nanoprecursors Produced by an Easily Scalable and Environmentally Friendly Process. Nanomaterials. 2020; 10(12):2533. https://doi.org/10.3390/nano10122533
Chicago/Turabian StyleAmaveda, Hippolyte, Maria A. Madre, Mario Mora, Miguel A. Torres, and Andres Sotelo. 2020. "Reduction in Processing Time in Ca3Co4O9+δ Ceramics through Nanoprecursors Produced by an Easily Scalable and Environmentally Friendly Process" Nanomaterials 10, no. 12: 2533. https://doi.org/10.3390/nano10122533