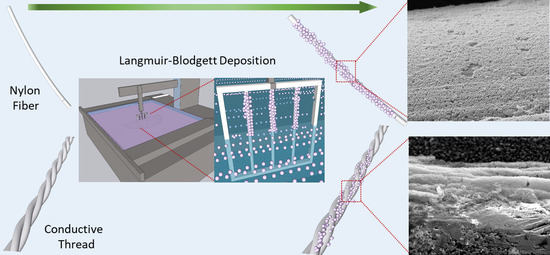
Coating of Conducting and Insulating Threads with Porous MOF Particles through Langmuir-Blodgett Technique
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoskins, B.F.; Robson, R. Infinite Polymeric Frameworks Consisting of 3 Dimensionally Linked Rod-Like Segments. J. Am. Chem. Soc. 1989, 111, 5962–5964. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, P.; Wang, C.H.; Kaneti, Y.V.; Eguchi, M.; Lin, J.J.; Yamauchi, Y.; Na, J. Practical MOF Nanoarchitectonics: New Strategies for Enhancing the Processability of MOFs for Practical Applications. Langmuir 2020, 36, 4231–4249. [Google Scholar] [CrossRef] [PubMed]
- Dalstein, O.; Gkaniatsou, E.; Sicard, C.; Sel, O.; Perrot, H.; Serre, C.; Boissiere, C.; Faustini, M. Evaporation-Directed Crack-Patterning of Metal-Organic Framework Colloidal Films and Their Application as Photonic Sensors. Angew. Chem. Int. Ed. 2017, 56, 14011–14015. [Google Scholar] [CrossRef]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.L.; Li, J. Designer Metal-Organic Frameworks for Size-Exclusion-Based Hydrocarbon Separations: Progress and Challenges. Adv. Mater. 2020, 2002603. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, W.S. 2D Metal-Organic Framework Materials for Membrane-Based Separation. Adv. Mater. Interfaces 2020, 7, 1901514. [Google Scholar] [CrossRef]
- Couck, S.; Van Assche, T.R.; Liu, Y.Y.; Baron, G.V.; Van der Voort, P.; Denayer, J.F. Adsorption and Separation of Small Hydrocarbons on the Flexible, Vanadium-Containing MOF, COMOC-2. Langmuir 2015, 31, 5063–5070. [Google Scholar] [CrossRef]
- McKinlay, A.C.; Morris, R.E.; Horcajada, P.; Ferey, G.; Gref, R.; Couvreur, P.; Serre, C. BioMOFs: Metal-Organic Frameworks for Biological and Medical Applications. Angew. Chem. Int. Ed. 2010, 49, 6260–6266. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, G.; Li, Z.S.; Yuan, C.W.; Bai, Y.F.; Fu, D.G. Selective Electrochemical Detection of Dopamine on Polyoxometalate-Based Metal-Organic Framework and Its Composite with Reduced Graphene Oxide. Adv. Mater. Interfaces 2017, 4, 1601241. [Google Scholar] [CrossRef]
- Roy, K.; Jana, S.; Ghosh, S.K.; Mahanty, B.; Mallick, Z.; Sarkar, S.; Sinha, C.; Mandal, D. Three-Dimensional MOF-Assisted Self-Polarized Ferroelectret: An Effective Autopowered Remote Healthcare Monitoring Approach. Langmuir 2020, 36, 11477–11489. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, Q.Y.; Al-Enizi, A.M.; Nafady, A.; Ma, S.Q. Recent advances in MOF-based photocatalysis: Environmental remediation under visible light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Zhuang, J.L.; Terfort, A.; Woll, C. Formation of oriented and patterned films of metal-organic frameworks by liquid phase epitaxy: A review. Coordin. Chem. Rev. 2016, 307, 391–424. [Google Scholar] [CrossRef]
- Sanchez, E.P.V.; Knebel, A.; Sanchez, L.I.; Klumpp, M.; Woll, C.; Dittmeyer, R. Studying ZIF-8 SURMOF Thin Films with a Langatate Crystal Microbalance: Single-Component Gas Adsorption Isotherms Measured at Elevated Temperatures and Pressures. Langmuir 2020, 36, 8444–8450. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Sakamoto, K.; Inada, H.; Kawata, M.; Takasaki, G.; Imawaka, K. Vapor-Phase Synthesis of ZIF-8 MOF Thick Film by Conversion of ZnO Nanorod Array. Langmuir 2018, 34, 7028–7033. [Google Scholar] [CrossRef] [PubMed]
- Ohnsorg, M.L.; Beaudoin, C.K.; Anderson, M.E. Fundamentals of MOF Thin Film Growth via Liquid-Phase Epitaxy: Investigating the Initiation of Deposition and the Influence of Temperature. Langmuir 2015, 31, 6114–6121. [Google Scholar] [CrossRef]
- Lopez-Maya, E.; Montoro, C.; Rodriguez-Albelo, L.M.; Cervantes, S.D.A.; Lozano-Perez, A.A.; Cenis, J.L.; Barea, E.; Navarro, J.A.R. Textile/Metal-Organic-Framework Composites as Self-Detoxifying Filters for Chemical-Warfare Agents. Angew. Chem. Int. Ed. 2015, 54, 6790–6794. [Google Scholar] [CrossRef]
- Emam, H.E.; Abdelhameed, R.M. Anti-UV Radiation Textiles Designed by Embracing with Nano-MIL (Ti, In)-Metal Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 28034–28045. [Google Scholar] [CrossRef]
- Li, G.P.; Cao, F.; Zhang, K.; Hou, L.; Gao, R.C.; Zhang, W.Y.; Wang, Y.Y. Design of Anti-UV Radiation Textiles with Self-Assembled Metal-Organic Framework Coating. Adv. Mater. Interfaces 2020, 7, 1901525. [Google Scholar] [CrossRef]
- Smith, M.K.; Mirica, K.A. Self-Organized Frameworks on Textiles (SOFT): Conductive Fabrics for Simultaneous Sensing, Capture, and Filtration of Gases. J. Am. Chem. Soc. 2017, 139, 16759–16767. [Google Scholar] [CrossRef]
- Tahghighi, M.; Janner, D.; Ignes-Mullol, J. Optimizing Gold Nanoparticle Size and Shape for the Fabrication of SERS Substrates by Means of the Langmuir-Blodgett Technique. Nanomaterials 2020, 10, 2264. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Nova, D.; Teixeira, M.; Cardoso, V.; Morgado, P.; Nunes, B.; Colaco, R.; Faure, M.C.; Fontaine, P.; Goldmann, M.; et al. Langmuir Films of Perfluorinated Fatty Alcohols: Evidence of Spontaneous Formation of Solid Aggregates at Zero Surface Pressure and Very Low Surface Density. Nanomaterials 2020, 10, 2257. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Pearson, C.; Molloy, A.; Cousins, M.A.; Green, M.; Kolliopoulou, S.; Dimitrakis, P.; Normand, P.; Tsoukalas, D.; Petty, M.C. Langmuir-Blodgett film deposition of metallic nanoparticles and their application to electronic memory structures. Nano Lett. 2003, 3, 533–536. [Google Scholar] [CrossRef]
- Reculusa, S.; Ravaine, S. Synthesis of colloidal crystals of controllable thickness through the Langmuir-Blodgett technique. Chem. Mater. 2003, 15, 598–605. [Google Scholar] [CrossRef]
- Kohoutek, T.; Parchine, M.; Bardosova, M.; Pemble, M.E. Controlled self-assembly of Langmuir-Blodgett colloidal crystal films of monodispersed silica particles on non-planar substrates. Colloids Surface A 2020, 593, 124625. [Google Scholar] [CrossRef]
- Benito, J.; Sorribas, S.; Lucas, I.; Coronas, J.; Gascon, I. Langmuir-Blodgett Films of the Metal-Organic Framework MIL-101(Cr): Preparation, Characterization, and CO2 Adsorption Study Using a QCM-Based Setup. ACS Appl. Mater. Interfaces 2016, 8, 16486–16492. [Google Scholar] [CrossRef] [Green Version]
- Navarro, M.; Benito, J.; Paseta, L.; Gascon, I.; Coronas, J.; Tellez, C. Thin-Film Nanocomposite Membrane with the Minimum Amount of MOF by the Langmuir-Schaefer Technique for Nanofiltration. ACS Appl. Mater. Interfaces 2018, 10, 1278–1287. [Google Scholar] [CrossRef]
- Andres, M.A.; Benzaqui, M.; Serre, C.; Steunou, N.; Gascon, I. Fabrication of ultrathin MIL-96(Al) films and study of CO2 adsorption/desorption processes using quartz crystal microbalance. J. Colloid Interf. Sci. 2018, 519, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Andres, M.A.; Vijjapu, M.T.; Surya, S.G.; Shekhah, O.; Salama, K.N.; Serre, C.; Eddaoudi, M.; Roubeau, O.; Gascon, I. Methanol and Humidity Capacitive Sensors Based on Thin Films of MOF Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 4155–4162. [Google Scholar] [CrossRef]
- Rauf, S.; Vijjapu, M.T.; Andres, M.A.; Gascon, I.; Roubeau, O.; Eddaoudi, M.; Salama, K.N. Highly Selective Metal-Organic Framework Textile Humidity Sensor. ACS Appl. Mater. Interfaces 2020, 12, 29999–30006. [Google Scholar] [CrossRef]
- Benzaqui, M.; Pillai, R.S.; Sabetghadam, A.; Benoit, V.; Normand, P.; Marrot, J.; Menguy, N.; Montero, D.; Shepard, W.; Tissot, A.; et al. Revisiting the Aluminum Trimesate-Based MOF (MIL-96): From Structure Determination to the Processing of Mixed Matrix Membranes for CO2 Capture. Chem. Mater. 2017, 29, 10326–10338. [Google Scholar] [CrossRef] [Green Version]
- Owyeung, R.E.; Panzer, M.J.; Sonkusale, S.R. Colorimetric Gas Sensing Washable Threads for Smart Textiles. Sci. Rep. UK 2019, 9, 5607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kommula, V.P.; Reddy, K.O.; Shukla, M.; Marwalad, T.; Reddy, E.V.S.; Rajulu, A.V. Extraction, modification, and characterization of natural ligno-cellulosic fiber strands from napier grass. Int. J. Polym. Anal. Charact. 2016, 21, 18–28. [Google Scholar] [CrossRef]
- Haney, C.I.; Martin, M.E.; Monroe, A.T. Cellulose Purification. U.S. Patent 2408849A, 8 October 1946. [Google Scholar]
- Börjesson, M.; Westman, G. Crystalline Nanocellulose—Preparation, Modification, and Properties. In Cellulose Fundamental Aspects and Current Trends; Poletto, M., Ed.; IntechOpen: London, UK, 2014. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauf, S.; Andrés, M.A.; Roubeau, O.; Gascón, I.; Serre, C.; Eddaoudi, M.; Salama, K.N. Coating of Conducting and Insulating Threads with Porous MOF Particles through Langmuir-Blodgett Technique. Nanomaterials 2021, 11, 160. https://doi.org/10.3390/nano11010160
Rauf S, Andrés MA, Roubeau O, Gascón I, Serre C, Eddaoudi M, Salama KN. Coating of Conducting and Insulating Threads with Porous MOF Particles through Langmuir-Blodgett Technique. Nanomaterials. 2021; 11(1):160. https://doi.org/10.3390/nano11010160
Chicago/Turabian StyleRauf, Sakandar, Miguel A. Andrés, Olivier Roubeau, Ignacio Gascón, Christian Serre, Mohamed Eddaoudi, and Khaled N. Salama. 2021. "Coating of Conducting and Insulating Threads with Porous MOF Particles through Langmuir-Blodgett Technique" Nanomaterials 11, no. 1: 160. https://doi.org/10.3390/nano11010160