Hybrid Nanostructures Obtained by Transport and Condensation of Tungsten Oxide Vapours onto CNW Templates
Abstract
:1. Introduction
2. Materials and Methods
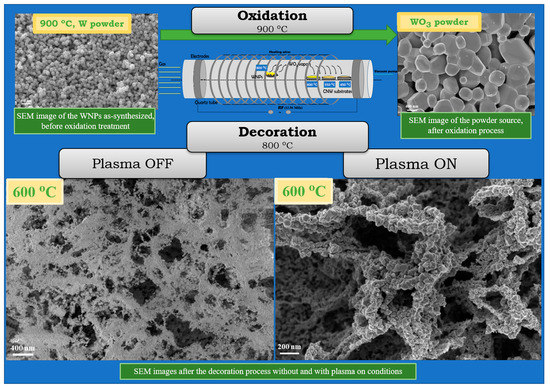
2.1. Preparation of Tungsten Nanoparticles, Carbon Nanowalls and Their Hybrid Architectures
2.1.1. Synthesis of CNW Nanostructures
2.1.2. Synthesis of Tungsten Nanoparticles
2.1.3. Synthesis of CNW/WO3 Hybrid Nanostructures
2.2. Material Characterization
3. Results and Discussion
3.1. Properties of the Tungsten Oxide Particles
3.2. Characterization of the Hybrid Nanostructures Synthesized in the Absence of Plasma (PLASMA-OFF)
3.2.1. Morphological Aspects
3.2.2. Chemical Composition
3.3. Characterization of the Hybrid Nanostructures Synthesized in Presence of Plasma (PLASMA-ON)
3.3.1. Morphological Aspects
3.3.2. Chemical Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vizireanu, S.; Mitu, B.; Dinescu, G. Nanostructured carbon growth by expanding RF plasma assisted CVD on Ni-coated silicon substrate. Surf. Coat. Technol. 2005, 200, 1132–1136. [Google Scholar] [CrossRef]
- Vizireanu, S.; Mitu, B.; Dinescu, G.; Nistor, L.; Ghica, C.; Maraloiu, A.; Stancu, M.; Ruxandra, G. Varieties of nanostructured carbon grown by expanding radiofrequency plasma beam. J. Optoelectron. Adv. Mater. 2007, 9, 1649–1652. [Google Scholar]
- Vizireanu, S.; Nistor, L.; Haupt, M.; Katzenmaier, V.; Oehr, C.; Dinescu, G. Carbon nanowalls growth by radiofrequency plasma-beam-enhanced chemical vapor deposition. Plasma Process Polym. 2008, 5, 263–268. [Google Scholar] [CrossRef]
- Vizireanu, S.; Stoica, S.D.; Mitu, B.; Husanu, M.A.; Galca, A.; Nistor, L.; Dinescu, G. Radiofrequency plasma beam deposition of various forms of carbon based thin films and their characterization. Appl. Surf. Sci. 2009, 255, 5378–5381. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Vizireanu, S.; Dinescu, G.; Blanco, C.; Santamaria, R. Carbon nanowalls thin films as nanostructured electrode materials in vanadium redox flow batteries. Nano Energy 2012, 1, 833–839. [Google Scholar] [CrossRef]
- Dinh, T.M.; Achour, A.; Vizireanu, S.; Dinescu, G.; Nistor, L.; Armstrong, K.; Guaye, D.; Pecha, D. Hydrous RuO2 /carbon nanowalls hierarchical structures for all-solid-state ultrahigh-energy-density microsupercapacitors. Nano Energy 2014, 10, 288–294. [Google Scholar] [CrossRef]
- Suetin, N.V.; Evlashin, S.A.; Egorov, A.V.; Mironovich, K.V.; Dagesyan, S.A.; Yashina, L.V.; Goodilin, E.A.; Krivchenko, V.A. Self-assembled nanoparticle patterns on carbon nanowall surfaces. Phys. Chem. Chem. Phys. 2016, 18, 12344–12349. [Google Scholar] [CrossRef]
- Krivchenko, V.A.; Itkis, D.M.; Evlashin, S.A.; Semenenko, D.A.; Goodilin, E.A.; Rakhimov, A.T.; Voronin, P.V. Carbon nanowalls decorated with silicon for lithium-ion batteries. Carbon 2012, 50, 1438–1442. [Google Scholar] [CrossRef]
- Wu, Y.H.; Yang, B.J.; Han, G.C.; Zong, B.Y.; Ni, H.Q.; Luo, P.; Shen, Z.X. Fabrication of a Class of Nanostructured Materials Using Carbon Nanowalls as the Templates. Adv. Funct. Mater. 2002, 12, 489–494. [Google Scholar] [CrossRef]
- Wang, H.; Quan, X.; Yu, H.; Chen, S. Fabrication of a TiO2/carbon nanowall heterojunction and its photocatalytic ability. Carbon 2008, 46, 1126–1132. [Google Scholar] [CrossRef]
- Yang, B.; Wu, Y.; Zong, B.; Shen, Z. Electrochemical Synthesis and Characterization of Magnetic Nanoparticles on Carbon Nanowall Templates. Nano Lett. 2002, 2, 751–754. [Google Scholar] [CrossRef]
- Vuong, N.M.; Trung, T.N.; Hien, T.T.; Chinh, N.D.; Quang, N.D.; Lee, D.; Kim, D. Ni2O3 Decoration of WO3 Thin Film for High Sensitivity NH3 Gas Sensor. Mater. Trans. 2015, 56, 1354–1357. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Chong, M.N.; Chan, E.S. Nanostructured Tungsten Trioxide Thin Films Synthesized for Photoelectrocatalytic Water Oxidation: A review. ChemSusChem 2014, 7, 2974–2997. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.; Mathew, J.; George, S.C. Nanomaterials for photoelectrochemical water splitting—Review. Int. J. Hydrogen Energy 2018, 43, 4804–4817. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; Monge, M.A.; Pérez, P. On the oxidation mechanism of pure tungsten in the temperature range 600–800 °C. Corros. Sci. 2012, 57, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Stoica, S.D.; Vizireanu, S.; Acsente, T.; Dinescu, G. Hybrid Nanomaterial Architectures: Combining Layers of Carbon Nanowalls, Nanotubes, and Particles. Plasma Chem. Plasma Process 2018, 38, 695–706. [Google Scholar] [CrossRef]
- Acsente, T.; Negrea, R.F.; Nistor, L.C.; Logofatu, C.; Matei, E.; Birjega, R.; Grisolia, C.; Dinescu, G. Synthesis of flower-like tungsten nanoparticles by magnetron sputtering combined with gas aggregation. Eur. Phys. J. D 2015, 69, 7. [Google Scholar] [CrossRef]
- Acsente, T.; Negrea, R.F.; Nistor, L.C.; Matei, E.; Grisolia, C.; Birjega, R.; Dinescu, G. Tungsten nanoparticles with controlled shape and crystallinity obtained by magnetron sputtering and gas aggregation. Mater. Lett. 2017, 200, 121–124. [Google Scholar] [CrossRef]
- Wang, H.; Su, Y.; Chen, S.; Quan, X. Growth of tungsten oxide on carbon nanowalls templates. Mater. Res. Bull. 2013, 48, 1304–1307. [Google Scholar] [CrossRef]
- Szkutnik, P.D.; Angélidès, L.; Todorova, V.; Jiménez, C. Qualification of a sublimation tool applied to the case of metalorganic chemical vapor deposition of In2O3 from In(tmhd)3 as a solid precursor. Rev. Sci. Instrum. 2016, 87, 023901. [Google Scholar] [CrossRef]
- Jeng, G.D.; Mitchell, J.W.; Seibles, L. Method for Growing Continous Diamond Films. U.S. Patent 5,441,013A, 15 August 1995. [Google Scholar]
- Tung, H.-Y.; Guan, Z.-Y.; Liu, T.-Y.; Chen, H.-Y. Vapor sublimation and deposition to build porous particles and composites. Nat. Commun. 2018, 9, 2564. [Google Scholar] [CrossRef]
- Mitu, B.; Bauer-Gogonea, S.; Leonhartsberger, H.; Lindner, M.; Bauer, S.; Dinescu, G. Plasma-deposited parylene-like thin films: Process and material properties. Surf. Coat. Technol. 2003, 174, 124–130. [Google Scholar] [CrossRef]
- Ma, S.; Hu, M.; Zeng, P.; Li, M.; Yan, W.; Qin, Y. Synthesis and low-temperature gas sensing properties of tungsten oxide nanowires/porous silicon composite. Sens. Actuators B Chem. 2014, 192, 341–349. [Google Scholar] [CrossRef]
- Wang, D.-F.; Liang, J.-R.; Li, C.-Q.; Yan, W.-J.; Hu, M. Room temperature NO2 gas sensing of Au-loaded tungsten oxide nanowires/porous silicon hybrid structure. Chin. Phys. B 2016, 25, 028102. [Google Scholar] [CrossRef]
- Ma, S.; Hu, M.; Li, M.; Liang, J.; Li, C. Synthesis of tungsten oxide nanowires/porous silicon composite and its sensing properties for NO2. In Proceedings of the 13th IEEE International Conference on Nanotechnology (IEEE-NANO 2013), Beijing, China, 5–8 August 2013; p. 14060524. [Google Scholar] [CrossRef]
- Sangiovanni, D.G.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Ab initio molecular dynamics of atomic-scale surface reactions: Insights into metal organic chemical vapor deposition of AlN on graphene. Phys. Chem. Chem. Phys. 2018, 20, 17751–17761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos, R.B.; Rivelino, R.; de Brito Mota, F.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Dopant species with Al-Si and N-Si bonding in the MOCVD of AlN implementing trimethylaluminum, ammonia and silane. J. Phys. D Appl. Phys. 2015, 48, 295104. [Google Scholar] [CrossRef]
- Vizireanu, S.; Stoica, S.D.; Luculescu, C.; Nistor, L.C.; Mitu, B.; Dinescu, G. Plasma techniques for nanostructured carbon materials synthesis. A case study: Carbon nanowall growth by low pressure expanding RF plasma. Plasma Sources Sci. Technol. 2010, 19, 034016. [Google Scholar] [CrossRef]
- Stoica, S.D.; Vizireanu, S.; Luculescu, C.R.; Mitu, B.; Dinescu, G. Metastable growth regime for carbon nanowalls and carbon nanofibers in an Ar/H2/C2H2 radiofrequency plasma jet. Plasma Sources Sci. Technol. 2020, 29, 105007. [Google Scholar] [CrossRef]
- Constantinescu, C.; Vizireanu, S.; Ion, V.; Aldica, G.; Stoica, S.D.; Lazea-Stoyanova, A.; Alloncle, A.P.; Delaporte, P.; Dinescu, G. Laser-induced forward transfer of carbon nanowalls for soft electrodes fabrication. Appl. Surf. Sci. 2016, 374, 49–55. [Google Scholar] [CrossRef]
- Habainy, J.; Nilsson, C. Oxidation of Pure Tungsten in the Temperature Interval 400° to 900 °C. Master’s Thesis, Faculty of Engineering Lund University, Lund, Sweden, September 2013. [Google Scholar]
- Vizireanu, S.; Ionita, M.D.; Dinescu, G.; Enculescu, I.; Baibarac, M.; Baltog, I. Post-synthesis Carbon Nanowalls Transformation under Hydrogen, Oxygen, Nitrogen, Tetrafluoroethane and Sulfur Hexafluoride Plasma Treatments. Plasma Process. Polym. 2012, 9, 363–370. [Google Scholar] [CrossRef]
- Zhdanov, V.P. Simulations of oxidation of metal nanoparticles with a grain boundary inside. React. Kinet. Mech. Catal. 2020, 130, 685–697. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Chang, C.-W. Preparation of Orthorhombic WO3 Thin Films and Their Crystal Quality-Dependent Dye, Photodegradation Ability. Coatings 2019, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Ham, H.; Park, N.H.; Kim, S.S.; Hyoun, W.K. Evidence of Ostwald ripening during evolution of micro-scale solid carbon spheres. Sci. Rep. 2014, 4, 3579. [Google Scholar] [CrossRef]
- Arnas, C.; Chami, A.; Couedel, L.; Acsente, T.; Cabie, M.; Neisius, T. Thermal balance of tungsten monocrystalline nanoparticles in high pressure magnetron discharges. Phys. Plasmas 2019, 26, 053706. [Google Scholar] [CrossRef]
- Warren, A.; Nylund, A.; Olefjord, I. Oxidation of tungsten and tungsten carbide in dry and humid atmospheres. Int. J. Refract. Met. Hard Mater. 1996, 14, 345–353. [Google Scholar] [CrossRef]
- Marascu, V.; Stancu, C.; Satulu, V.; Bonciu, A.; Grisolia, C.; Dinescu, G. Material Erosion and Dust Formation during Tungsten Exposure to Hollow-Cathode and Microjet Discharges. Appl. Sci. 2020, 10, 6870. [Google Scholar] [CrossRef]
- Marascu, V.; Lazea–Stoyanova, A.; Bonciu, A.; Satulu, V.; Dinescu, G. Tungsten particles fabrication by a microjet discharge. Mater. Res. Express 2020, 7, 066509. [Google Scholar] [CrossRef]
- Chiu, H.-T.; Chuang, S.-H. Tungsten nitride thin films prepared by MOCVD. J. Mater. Res. 1993, 8, 1353–1360. [Google Scholar] [CrossRef] [Green Version]
- Bchir, O.J.; Kim, K.C.; Anderson, T.J.; Craciun, V.; Brooks, B.C.; McElwee-White, L. Effect of NH3 on Film Properties of MOCVD Tungsten Nitride from Cl4(CH3CN)W(NiPr). J. Electrochem. Soc. 2004, 151, G697. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Baek, D.-H.; Pyo, S.; Kim, J. Carbon-Doped WO3 Nanostructure Based on CNT Sacrificial Template and its Application to Highly Sensitive NO2 Sensor. IEEE Sens. J. 2020, 20, 5705–5711. [Google Scholar] [CrossRef]
















| C1s (%) | O1s (%) | N1s (%) | W4f (%) | |
|---|---|---|---|---|
| CNW control sample/after synthesis | 92.19 | 4.99 | 2.83 | - |
| CNW after thermal treatment (No powder source) (550 °C) | 58.48 | 38.2 | 3.32 | - |
| CNW surfaces obtained after WO3 decoration (550 °C) | 26.39 | 45.77 | 23.51 | 4.33 |
| C1s (%) | O1s (%) | N1s (%) | W4f (%) | |
|---|---|---|---|---|
| CNW control sample/after synthesis | 92.19 | 4.99 | 2.83 | |
| CNW after thermal treatment (No powder source) (550 °C) | 51.78 | 44.21 | 4.01 | |
| CNW surfaces obtained after WO3 decoration (550 °C) | 20.05 | 49.87 | 23.43 | 6.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpen, L.G.; Acsente, T.; Sătulu, V.; Matei, E.; Vizireanu, S.; Biță, B.I.; Dinescu, G. Hybrid Nanostructures Obtained by Transport and Condensation of Tungsten Oxide Vapours onto CNW Templates. Nanomaterials 2021, 11, 835. https://doi.org/10.3390/nano11040835
Carpen LG, Acsente T, Sătulu V, Matei E, Vizireanu S, Biță BI, Dinescu G. Hybrid Nanostructures Obtained by Transport and Condensation of Tungsten Oxide Vapours onto CNW Templates. Nanomaterials. 2021; 11(4):835. https://doi.org/10.3390/nano11040835
Chicago/Turabian StyleCarpen, Lavinia Gabriela, Tomy Acsente, Veronica Sătulu, Elena Matei, Sorin Vizireanu, Bogdan Ionuț Biță, and Gheorghe Dinescu. 2021. "Hybrid Nanostructures Obtained by Transport and Condensation of Tungsten Oxide Vapours onto CNW Templates" Nanomaterials 11, no. 4: 835. https://doi.org/10.3390/nano11040835