Exploration of Copper Oxide Nanoneedle Electrosynthesis Applied in the Degradation of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
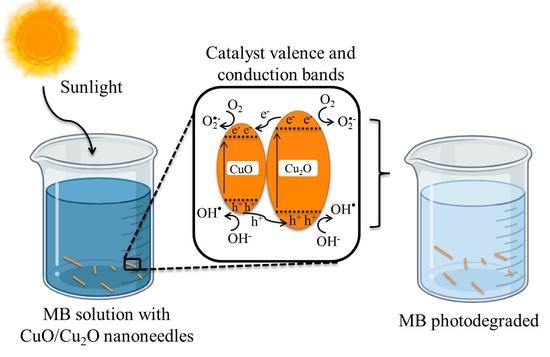
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boulett, A.; Pizarro, G.; Martín-Trasanco, R.; Sánchez, J.; Tasca, F.; Linarez, O.; Tello, A.; Oyarzún, D.P. Electrodeposition of Cu2O nanostructures with improved semiconductor properties. Cogent Eng. 2021, 8, 1875534. [Google Scholar] [CrossRef]
- Wong, M.H.; Berenov, A.; Qi, X.; Kappers, M.J.; Barber, Z.H.; Illy, B.; Lockman, Z.; Ryan, M.P.; MacManus-Driscoll, J.L. Electrochemical growth of ZnO nano-rods on polycrystalline Zn foil. Nanotechnology 2003, 14, 968–973. [Google Scholar] [CrossRef]
- Gao, P.; Wang, Z.L. Self-assembled nanowire−nanoribbon junction arrays of ZnO. J. Phys. Chem. B 2002, 106, 12653–12658. [Google Scholar] [CrossRef]
- Roy, S.; Basu, S. Improved zinc oxide film for gas sensor applications. Bull. Mater. Sci. 2002, 25, 513–515. [Google Scholar] [CrossRef]
- Sumida, T.; Wada, Y.; Kitamura, T.; Yanagida, S. Macroporous ZnO films electrochemically prepared by templating of opal films. Chem. Lett. 2001, 30, 38–39. [Google Scholar] [CrossRef]
- Holland, B.T.; Blanford, C.F.; Stein, A. Synthesis of macroporous minerals with highly ordered three-dimensional arrays of spheroidal voids. Science 1998, 281, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Selforganized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Allam, N.K.; Grimes, C.A. Effect of rapid infrared annealing on the photoelectrochemical properties of anodically fabricated TiO2 nanotube arrays. J. Phys. Chem. C 2009, 113, 7996–7999. [Google Scholar] [CrossRef]
- Diaz, J.J.; Fryauf, D.M.; Cormia, R.D.; Zhang, M.M.; Samuels, K.; Stanley, R.; Kobayashi, N.P. Reflectometry–ellipsometry reveals thickness, growth rate, and phase composition in oxidation of copper. ACS Appl. Mater. Interfaces 2016, 8, 22337–22344. [Google Scholar] [CrossRef]
- Aiswarya, A.S.; Biju, V. Nanostructured CuO: Facile synthesis, optical absorption and defect dependent electrical conductivity. Mater. Sci. Semicond. Process. 2017, 68, 38–47. [Google Scholar] [CrossRef]
- Bayat, F.; Sheibani, S. Enhancement of photocatalytic activity of CuO-Cu2O heterostructures through the controlled content of Cu2O. Mater. Res. Bull. 2021, 145, 111561. [Google Scholar] [CrossRef]
- Musselman, K.P.; Wisnet, A.; Iza, D.C.; Hesse, H.C.; Scheu, C.; MacManus-Driscoll, J.L.; Schmidt-Mende, L. Strong efficiency improvements in ultra-low-cost inorganic nanowire solar cells. Adv. Energy Mater. 2010, 22, E254–E258. [Google Scholar] [CrossRef]
- Tello, A.; Boulett, A.; Sánchez, J.; Pizarro, G.; Soto, C.; Linarez, O.; Sanhueza, R.; Oyarzún, D.P. An unexplored strategy for synthesis of ZnO nanowire films by electrochemical anodization using an organic-based electrolyte. Morphological and optical properties characterization. Chem. Phys. Lett. 2021, 788, 138825. [Google Scholar] [CrossRef]
- Jeong, D.; Lee, J.; Hong, H.; Choi, D.; Cho, J.W.; Kim, S.K.; Nam, Y. Absorption mechanism and performance characterization of CuO nanostructured absorbers. Sol. Energy Mater. Sol. Cells 2017, 169, 270–279. [Google Scholar] [CrossRef]
- Hara, M.; Kondo, T.; Komoda, M.; Ikeda, S.; Kondo, J.N.; Domen, K.; Michikazu, H.; Shinohara, K.; Tanaka, A. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chem. Commun. 1998, 3, 357–358. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Vanmaekelbergh, D.; Kelly, J.J. Cu2O: A catalyst for the photochemical decomposition of water. Chem. Commun. 1999, 12, 1069–1070. [Google Scholar] [CrossRef]
- Erné, B.; Vanmaekelbergh, D.; Kelly, J. Porous etching: A means to enhance the photoresponse of indirect semiconductors. Adv. Mater. 1995, 7, 739–742. [Google Scholar] [CrossRef]
- Van de Lagemaat, J.; Plakman, M.; Vanmaekelbergh, D.; Kelly, J. Enhancement of the light-to-current conversion efficiency in an n-SiC/solution diode by porous etching. Appl. Phys. Lett. 1996, 69, 2246–2248. [Google Scholar] [CrossRef]
- Rakhshani, A. Preparation, characteristics and photovoltaic properties of cuprous oxide a review. Solid State Electron. 1986, 29, 7–17. [Google Scholar] [CrossRef]
- Rai, B. Cu2O solar cells: A review. Sol. Cells 1988, 25, 265–272. [Google Scholar] [CrossRef]
- Martin, C. Nanomaterials: A membrane-based synthetic approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef]
- Sapp, S.; Lakshimi, B.; Martin, C. Template synthesis of bismuth telluride nanowires. Adv. Mater. 1999, 11, 402–404. [Google Scholar] [CrossRef]
- Tan, Y.; Xue, X.; Peng, Q.; Zhao, H.; Wang, T.; Li, Y. Controllable fabrication and electrical performance of single crystalline Cu2O nanowires with high aspect ratios. Nano Lett. 2007, 7, 3723–3728. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Li, H.; Qu, Z.; Fan, S.; Ji, M. Hierarchical growth of Cu2O double tower-tip-like nanostructures in water/oil microemulsion. Cryst. Growth Des. 2007, 7, 820–824. [Google Scholar] [CrossRef]
- McShane, C.; Choi, K.S. Photocurrent enhancement of n-type Cu2O electrodes achieved by controlling dendritic branching growth. J. Am. Chem. Soc. 2009, 131, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Allam, N.; Grimes, C. Effect of cathode material on the morphology and photoelectrochemical properties of vertically oriented TiO2 nanotube array. Sol. Energy Mater. Sol. Cells 2008, 92, 1468–1475. [Google Scholar] [CrossRef]
- Han, J.; Fan, F.; Xu, C.; Lin, S.; Wei, M.; Duan, X.; Wang, Z. ZnO nanotube-based dye-sensitized solar cell and its application in self-powered devices. Nanotechnology 2010, 21, 405203. [Google Scholar] [CrossRef]
- Allam, N.; Grimes, C. Formation of vertically oriented TiO2 nanotube arrays using a fluoride free HCl aqueous electrolyte. J. Phys. Chem. C 2007, 111, 13028–13032. [Google Scholar] [CrossRef]
- Allam, N.; Feng, X.; Grimes, C. Self-assembled fabrication of vertically oriented Ta2O5 nanotube arrays, and membranes thereof, by one-step tantalum anodization. Chem. Mater. 2008, 20, 6477–6481. [Google Scholar] [CrossRef]
- Allam, N.; El-Sayed, M. Photoelectrochemical water oxidation characteristics of anodically fabricated TiO2 nanotube arrays: Structural and optical properties. J. Phys. Chem. C 2010, 114, 12024–12029. [Google Scholar] [CrossRef]
- Wu, X.; Bai, H.; Zhang, J.; Chen, F.; Shi, G. Copper hydroxide nanoneedle and nanotube arrays fabricated by anodization of copper. J. Phys. Chem. B 2005, 109, 22836–22842. [Google Scholar] [CrossRef]
- Allam, N.K.; Shankar, K.; Grimes, C.A. Photoelectrochemical and water photoelectrolysis properties of ordered TiO2 nanotubes fabricated by tianodization in fluoride-free HCl electrolytes. J. Mater. Chem. 2008, 18, 2341–2348. [Google Scholar] [CrossRef]
- Allam, N.K.; Alamgir, F.; El-Sayed, M.A. Enhanced photoassisted water electrolysis using vertically oriented anodically fabricated Ti-Nb-Zr-O mixed oxide nanotube arrays. ACS Nano 2010, 4, 5819–5826. [Google Scholar] [CrossRef]
- Allam, N.K.; Shankar, K.; Grimes, C.A. A general method for the anodic formation of crystalline metal oxide nanotube arrays without the use of thermal annealing. Adv. Mater. 2008, 20, 3942–3946. [Google Scholar] [CrossRef]
- Nanjo, H.; Hassan, F.M.; Venkatachalam, S.; Teshima, N.; Kawasaki, K.; Aizawa, T.; Aida, T.; Ebina, T. Fabrication of nanostructured titania on flexible substrate by electrochemical anodization. J. Power Sources 2010, 195, 5902–5908. [Google Scholar] [CrossRef]
- Kandalkar, S.G.; Dhawale, D.S.; Kim, C.K.; Lokhande, C.D. Chemical synthesis of cobalt oxide thin film electrode for supercapacitor application. Synth. Met. 2010, 160, 1299–1302. [Google Scholar] [CrossRef]
- Oyarzún, D.P.; López, M.; Ramos, W.; Linarez, O.; Sánchez, J.; Pizarro, G.; Acosta, G.; Flores, M.; Arratia-Perez, R. Nanostructuring of anodic copper oxides in fluoride-containing ethylene glycol media. J. Electroanal. Chem. 2017, 807, 181–186. [Google Scholar] [CrossRef]
- Stepniowski, W.; Misiolk, W. Review of fabrication methods, physical properties, and applications of nanostructured copper oxides formed via electrochemical oxidation. Nanomaterials 2018, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Yuan, S.; Liang, B.; Li, G.; Pehkoen, S.; Zhang, T. Superhydrophobic CuO nanoneedle-covered copper surfaces for anticorrosion. J. Mater. Chem. A 2015, 3, 4374–4388. [Google Scholar] [CrossRef]
- Allam, N.; Grimes, C. Electrochemical fabrication of complex copper oxide nanoarchitectures via copper anodization in aqueous and non-aqueous electrolytes. Mater. Lett. 2011, 65, 1949–1955. [Google Scholar] [CrossRef]
- Momeni, M.; Ghayeb, Y.; Menati, M. Facile and green synthesis of CuO nanoneedles with high photo catalytic activity. J. Mater. Sci. Mater. Electron. 2016, 27, 9454–9460. [Google Scholar] [CrossRef]
- Singh, V.; Bansal, P. Fabrication and characterization of needle shaped CuO nanoparticles and their application as photocatalyst for degradation of organic pollutants. Mater. Lett. 2020, 261, 126929. [Google Scholar] [CrossRef]
- Tichapondwa, S.; Newman, J.; Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth 2020, 118–119, 102900. [Google Scholar] [CrossRef]
- Mittal, H.; Alhassan, S.M.; Ray, S.S. Efficient organic dye removal from wastewater by magnetic carbonaceous adsorbent prepared from corn starch. J. Environ. Chem. Eng. 2018, 6, 7119–7131. [Google Scholar] [CrossRef]
- Oyarce, E.; Pizarro, G.; Oyarzún, D.P.; Martín-Trasanco, R.; Sánchez, J. Adsorption of methylene blue in aqueous solution using hydrogels based on T 2-hydroxyethyl methacrylate copolymerized with itaconic acid or acrylic acid. Mater. Today Commun. 2020, 25, 101324. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Huang, C.; Lai, C.; Zhang, C.; Zeng, G.; Huang, D.; Cheng, M.; Hu, L.; Xiong, W.; Chen, M.; Wang, J.; et al. Semiconductor/boron nitride composites: Synthesis, properties, and photocatalysis applications. Appl. Catal. B Environ. 2018, 238, 6–18. [Google Scholar] [CrossRef]
- Flores, M.; Donoso, S.; Ortiz, M.; Fernández, H. Alkanethiol self-assembled monolayer on copper polycrystalline thin films: Influence on the resistivity. Mater. Chem. Phys. 2018, 208, 97. [Google Scholar] [CrossRef]
- Cabello, G.; Lillo, L.; Caro, C.; Buono-Cuore, B.; Chornik, B.; Flores, M.; Carrasco, C.; Rodriguez, C. Photochemical synthesis of AZrO3−X thin films (A=Ba, Ca and Sr) and their characterization. Ceram. Int. 2014, 40, 7761. [Google Scholar] [CrossRef]
- Benito, N.; Flores, M. Evidence of Mixed Oxide Formation on the Cu/SiO2 Interface. J. Phys. Chem. 2017, 121, 18771. [Google Scholar] [CrossRef]
- Oyarzún, D.P.; Córdova, R.; Linarez, O.; Muñoz, E.; Henríquez, R.; López, M.; Gomez, H. Morphological, electrochemical and photoelectrochemical characterization of nanotubular TiO2 synthetized electrochemically from different electrolytes. J. Solid State Electrochem. 2011, 15, 2265–2275. [Google Scholar] [CrossRef]
- Mayer, S.T.; Muller, R.H. An in situ raman spectroscopy study of the anodic oxidation of copper in alkaline media. J. Electrochem. Soc. 1992, 139, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Ivanda, M.; Waasmaier, D.; Endriss, A.; Ihringer, J.; Kirfel, A.; Kiefer, W. Low-temperature anomalies of cuprite observed by Raman spectroscopy and x-ray powder diffraction. J. Raman Spectrosc. 1997, 28, 487–493. [Google Scholar] [CrossRef]
- Platzman, I.; Brener, R.; Haick, H.; Tannenbaum, R. Oxidation of polycrystalline copper thin films at ambient conditions. J. Phys. Chem. C 2008, 112, 1101–1108. [Google Scholar] [CrossRef]
- Biesinger, M. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Oyarzún, D.P.; Broens, M.; Linarez, O.; López, M.; Islas, R.; Arratia-Perez, R. Simple and rapid one-step electrochemical synthesis of nanogranular Cu2O films. Chem. Select 2018, 3, 8610–8614. [Google Scholar] [CrossRef] [Green Version]
- Scuderi, V.; Amiard, G.; Boninelli, S.; Scalese, S.; Miritello, M.; Sberna, P.; Impellizzeri, G.; Privitera, V. Photocatalytic activity of CuO and Cu2O nanowires. Mater. Sci. Semicond. Process. 2016, 42, 89–93. [Google Scholar] [CrossRef]
- Dariani, R.; Esmaeili, A.; Mortezaali, A.; Dehghanpour, H. Photocatalytic reaction and degradation of methylene blue on TiO2 nano-sized particles. Optik 2016, 127, 7143–7154. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Wang, P. Hierarchical top-porous/bottom-tubular TiO2 nanostructures decorated with Pd nanoparticles for efficient photoelectrocatalytic decomposition of synergistic pollutants. ACS Appl. Mater. Interfaces 2012, 4, 900–996. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, A.; Nezamzadeh-Ejhieh, A. α-Fe2O3/Cu2O heterostructure: Brief characterization and kinetic aspect of degradation of methylene blue. Phys. Rev. B Condens. Matter 2020, 599, 412422. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Z.; Zhang, S.; Cui, Y.; Gao, S.; Wang, Y. Hydrothermal deposition of Cu2O-Ag nanoparticles co-sensitized TiO2 nanotube arrays and their enhanced photoelectrochemical performance. Sep. Purif. Technol. 2019, 211, 866–872. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, H.; Zhang, Z.; Wang, W. The Z-scheme heterojunction between TiO2 nanotubes and Cu2O nanoparticles mediated by Ag nanoparticles for enhanced photocatalytic stability and activity under visible light. Ceram. Int. 2018, 44, 22748–22759. [Google Scholar] [CrossRef]
- Li, X.; Raza, S.; Liu, C. Directly electrospinning synthesized Z-scheme heterojunction TiO2@Ag@Cu2O nanofibers with enhanced photocatalytic degradation activity under solar light irradiation. J. Environ. Chem. Eng. 2021, 9, 106133. [Google Scholar] [CrossRef]






| Cu-2p3/2 | |||
|---|---|---|---|
| Chemical Composition | eV | Area | %At Conc |
| Cu + Cu2O | 932.2 | 251,548 | 42 |
| CuO | 933.9 | 104,503 | 18 |
| Cu(OH)2 | 936.2 | 120,911 | 20 |
| Cu-Auger | |||
| Chemical Composition | eV | Area | %At Conc |
| Cu + CuO | 567.0 | 48,456 | 14 |
| Cu2O | 569.3 | 103,025 | 31 |
| O-1s | |||
| Chemical Composition | eV | Area | %At Conc |
| OH + Cu(OH)2 | 529.6 | 17,556 | 16 |
| Cu2O + CuO | 531.0 | 30,615 | 28 |
| H2O | 532.5 | 61,589 | 56 |
| Oxides | Copper Oxide Morphology | Copper Oxide Synthesis Type | Composition of the Electrolyte Solution | Application | Light Source | Degradation (%) | Dye | Refs. |
|---|---|---|---|---|---|---|---|---|
| Fe2O3/Cu2O | Nanoparticles | Hydrothermal | -- | Photocatalysis | Visible light | 90 | MB | [60] |
| Cu2O/Ag/TiO2 | 93 | MB | [61] | |||||
| Cu2O/Ag/TiO2 | electrodeposition | Cu(NO3)2 + NaOH + lactic acid | 98 | MB | [62] | |||
| Cu2O/Ag/TiO2/NFs PAN | Electrospinning | -- | 99 | MB | [63] | |||
| Cu2O/NFs PAN | 60 | MB | [63] | |||||
| P25-TiO2 (commercial standard) | -- | -- | UV light | 81 | MB | [43] | ||
| CuO | Nanoneedles | Solution chemistry in basic media | 95 | DR and VB | [42] | |||
| Cu2O/CuO | Chemical-thermal oxidation | Visible light | 80 | MB | [11] | |||
| CuO | Anodization and thermal treatment | KOH | Anticorrosive surface | -- | -- | -- | [39] | |
| CuO | Anodization and thermal treatment | KOH | -- | [31] | ||||
| Cu2O | Anodization | KOH | [40] | |||||
| CuO | KOH | Photocatalisis | Visible light | 93 | MO | [41] | ||
| Cu2O/CuO | KOH + 10% H2O + NH4F + ethylene glycol | Sunlight | 88 | MB | This study | |||
| Cu2O/CuO | NaOH + 5% H2O + NH4F + ethylene glycol | 82 | MB | This study | ||||
| Cu2O | NaOH + 1% H2O + NH4F + ethylene glycol | 74 | MB | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyarzún, D.P.; Tello, A.; Sánchez, J.; Boulett, A.; Linarez Pérez, O.E.; Martin-Trasanco, R.; Pizarro, G.d.C.; Flores, M.; Zúñiga, C. Exploration of Copper Oxide Nanoneedle Electrosynthesis Applied in the Degradation of Methylene Blue. Nanomaterials 2021, 11, 2994. https://doi.org/10.3390/nano11112994
Oyarzún DP, Tello A, Sánchez J, Boulett A, Linarez Pérez OE, Martin-Trasanco R, Pizarro GdC, Flores M, Zúñiga C. Exploration of Copper Oxide Nanoneedle Electrosynthesis Applied in the Degradation of Methylene Blue. Nanomaterials. 2021; 11(11):2994. https://doi.org/10.3390/nano11112994
Chicago/Turabian StyleOyarzún, Diego P., Alejandra Tello, Julio Sánchez, Andrés Boulett, Omar E. Linarez Pérez, Rudy Martin-Trasanco, Guadalupe del C. Pizarro, Marcos Flores, and César Zúñiga. 2021. "Exploration of Copper Oxide Nanoneedle Electrosynthesis Applied in the Degradation of Methylene Blue" Nanomaterials 11, no. 11: 2994. https://doi.org/10.3390/nano11112994