Unidirectional Magnetic Anisotropy in Molybdenum Dioxide–Hematite Mixed-Oxide Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
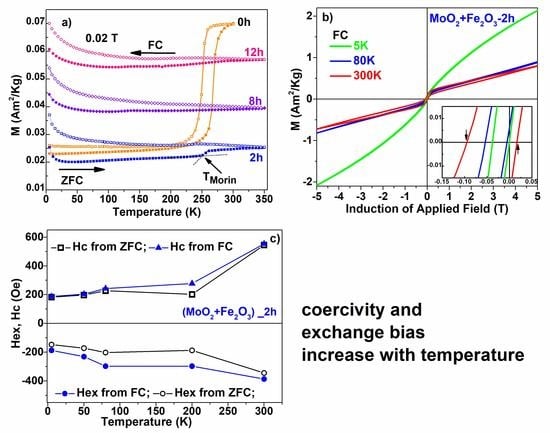
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, M.Y.; Xu, X.; Chen, G.; Zhong, Y.; Cai, R.; Li, L.; Shao, Z. Surfactant- Free Self-Assembly of Reduced Graphite Oxide-MoO2 Nanobelt Composites Used as Electrodes for Lithium Ion Batteries. Electrochim. Acta 2016, 211, 972–981. [Google Scholar] [CrossRef]
- Zhou, E.; Wang, C.; Zhao, Q.; Li, Z.; Shao, M.; Deng, X.; Liu, X.; Xu, X. Facile Synthesis of MoO2 Nanoparticles as High-Performance Supercapacitor Electrodes and Photocatalysts. Ceram. Int. 2016, 42, 2198–2203. [Google Scholar] [CrossRef]
- Zhang, T.; Kong, L.B.; Liu, M.C.; Dai, Y.H.; Yan, K.; Hu, B.; Luo, Y.C.; Kang, L. Design and Preparation of MoO2/MoS2 as Negative Electrode Materials for Supercapacitors. Mater. Des. 2016, 112, 88–96. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, Z.; Li, X.; Liang, J.; Zhu, Y.; Qian, Y. MoO2 Nanoparticles as High Capacity Intercalation Anode Material for Long Cycle Lithium Ion Battery. Electrochim. Acta 2016, 213, 416–422. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, P.; Dai, J.; Zhang, H.; Xie, A.; Shen, Y. Facile Synthesis and Electrochemical Properties of MoO2/Reduced Grapheme Oxide Hybrid for Efficient Anode of Lithium Ion Battery. Ceram. Int. 2016, 42, 3618–3624. [Google Scholar] [CrossRef]
- Wang, G.X.; Gou, X.L.; Horvat, J.; Park, J. Facile Synthesis and Characterization of Iron Oxide Semiconductor Nanowires for Gas Sensing Application. J. Phys. Chem. C 2008, 112, 15220–15225. [Google Scholar] [CrossRef]
- Raffaella, B.; Etienne, S.; Cinzia, G.; Fabia, G.; Mar, G.H.; Miguel, A.G.; Roberto, C.; Pantaleo, D.C. Colloidal Semiconductor/Magnetic Heterostructures Based on Iron-Oxide-Functionalized Brookite TiO2 Nanorods. Phys. Chem. Chem. Phys. 2009, 11, 3680–3691. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Rivas, J.A.; Amiridis, M.D. Catalytic Oxidation of 1,2-Dichlorobenzene over Supported Transition Metal Oxides. J. Catal. 2000, 193, 264–272. [Google Scholar] [CrossRef]
- Sorescu, M.; Diamandescu, L.; Tomescu, A.; Tarabasanu-Mihaila, D.; Teodorescu, V. Structure and Sensing Properties of 0.1SnO2-0.9Fe2O3 System. Mater. Chem. Phys. 2008, 107, 127–131. [Google Scholar] [CrossRef]
- Kuncser, V.; Schinteie, G.; Palade, P.; Mustata, I.; Lungu, C.P.; Stefan, N.; Chiriac, H.; Vladoiu, R.; Filoti, G. Spin Configurations and Interfacial Diffusion in Exchange Bias and Spin Valve Systems with Ir–Mn Antiferromagnetic Pinning Layers. Hyp. Int. 2009, 191, 135–141. [Google Scholar] [CrossRef]
- Meiklejohn, W.H.; Bean, C.P. New Magnetic Anisotropy. Phys. Rev. 1956, 102, 1413. [Google Scholar] [CrossRef]
- Radu, F.; Zabel, H. Exchange Bias Effect of Ferro-Antiferromagnetic Heterostructures. Magnetic Heterostructures, Chapter Springer Tracts. In Modern Physics Springer; Bader, S., Zabel, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 227, pp. 97–184. [Google Scholar]
- Nogues, J.; Schuller, I.K. Exchange Bias. J. Magn. Magn. Mater. 1992, 192, 203–232. [Google Scholar] [CrossRef]
- Kuncser, V.; Schinteie, G.A.; Kuncser, A.C.; Leca, A.; Scarisoreanu, M.; Morjan, I.; Filoti, G. Physical Mechanisms of Exchange Coupling Effects in Nanoparticulate Diluted Magnetic Oxides Obtained by Laser Pyrolysis. J. Phys. Chem. C 2017, 121, 9063–9069. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Z.W.; Zhang, Q.H.; Chen, S.R.; Jin, Q.; Yao, H.B.; Xu, S.; Meng, F.Q.; Yin, X.M.; Wang, C.; et al. Exchange Coupling in Synthetic Anion-Engineered Chromia Heterostructures. Adv. Funct. Mater. 2021, 32, 2109828. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, M.; Srinet, G.; Siqueiros, J.M.; Herrera, O.R. Structural, Raman analysis and exchange bias effects in Mn doped multiferroic Bi0.80La0.10Ca0.10Fe1−xMnxO3 ceramics. Ceram. Int. 2021, 47, 6834–6841. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.L.; Zhang, X.; Zhong, M.L.; Zhong, Z.C.; Rao, G.H. Tunable reversals of magnetization and exchange bias of perovskite YbCr1−xFexO3 (x = 0–0.15). Ceram. Int. 2019, 45, 6143–6148. [Google Scholar] [CrossRef]
- Dong, Y.N.; Zhao, X.N.; Han, X.; Fan, Y.B.; Xie, X.J.; Chen, Y.X.; Bai, L.H.; Dai, Y.Y.; Yan, S.S.; Tian, Y.F. Spin-orbit torque controllable complete spin logic in a single magnetic heterojunction. Appl. Phys. Lett. 2021, 118, 152403. [Google Scholar] [CrossRef]
- Guo, Y.; Ouyang, Y.; Sato, N.; Ooi, C.C.; Wang, S.X. Exchange-Biased Anisotropic Magnetoresistive Field Sensor. IEEE Sens. J. 2017, 17, 3309–3315. [Google Scholar] [CrossRef]
- Klug, M.J.; Thormahlen, L.; Robisch, V.; Toxvaerd, S.D.; Hoft, M.; Knochel, R.; Quandt, E.; Meyner, D.; McCord, J. Antiparallel exchange biased multilayers for low magnetic noise magnetic field sensors. Appl. Phys. Lett. 2019, 114, 192410. [Google Scholar] [CrossRef]
- Kazantseva, N.E.; Smolkova, I.S.; Babayan, V.; Vilcakova, J.; Smolka, P.; Saha, P. Magnetic Nanomaterials for Arterial Embolization and Hyperthermia of Parenchymal Organs Tumors: A Review. Nanomaterials 2021, 11, 3402. [Google Scholar] [CrossRef]
- Graca, M.P.; Soreto, S.; Gavinho, S.; Valente, M.; Salgueiro, C.; Nunes, J.; Soares, P.; Lanca, M.; Vieira, T.; Silva, J. Nanomaterials for magnetic hyperthermia. Eur. J. Public Health 2021, 31 (Suppl. 2), ckab120-066. [Google Scholar] [CrossRef]
- Trotta, R.; Tolea, F.; Valeanu, M.; Diamandescu, L.; Grabias, A.; Sorescu, M. Magnetic and Hyperfine Properties of Molibdenum Dioxide-Hematite Mixed Oxide Nanostructures. MRS Adv. 2018, 3, 2887–2892. [Google Scholar] [CrossRef]
- Knauss, M.; Tolea, F.; Valeanu, M.; Diamandescu, L.; Trotta, R.; Wood, K.; Grabias, A.; Sorescu, M.J. Mechanochemical Synthesis and Characterization of Molibdenum Dioxide-Hematite Nanostructures with Different Molarities. Min. Mat. Charact. Eng. 2018, 6, 587–600. [Google Scholar]
- Tolea, F.; Grecu, M.N.; Kuncser, V.; Constantinescu, S.G.; Ghica, D. On the role of Fe ions on magnetic properties of doped TiO2 nanoparticles. Appl. Phys. Lett. 2015, 106, 142404. [Google Scholar] [CrossRef]
- Nistor, S.V.; Stefan, M.; Nistor, L.C.; Kuncser, V.; Ghica, D.; Vlaicu, I.D. Aggregates of Mn2+ Ions in Mesoporous Self-Assembled Cubic ZnS:Mn Quantum Dots: Composition, Localization, Structure, and Magnetic Properties. J. Phys. Chem. C 2016, 120, 14454–14466. [Google Scholar] [CrossRef]
- Kuncser, V.E.; Stromberg, F.; Acet, M.; Keune, W. Mössbauer Effect Study of Correlation Between Structure and Exchange-Bias Effect In Ferromagnetic Fe∕Antiferromagnetic FeSn2FeSn2 Bilayers. J. Appl. Phys. 2005, 97, 063513. [Google Scholar] [CrossRef]
- Gupta, R.K.; Ghosh, K.; Dong, L.; Kahol, P.K. Structural and magnetic properties of nanostructured iron oxid. Phys. e-Low-Dimens. Syst. Nanostruct. 2011, 43, 1095–1098. [Google Scholar] [CrossRef]
- Kubaniova, D.; Kubickova, L.; Kmjec, T.; Zaveta, K.; Niznansky, D.; Brazda, P.; Klementova, M.; Kohout, J. Hematite: Morin temperature of nanoparticles with different size. J. Magn. Magn. Mater. 2019, 475, 611–619. [Google Scholar] [CrossRef]
- Nogués, J.; Schuller, I.K. Exchange bias. JMMM 1999, 192, 203–232. [Google Scholar] [CrossRef]
- Stromberg, F.; Keune, W.; Kuncser, V.; Westerholt, K. Fe Coverage Induced Out-of-plane Spin Component of the Antiferromagnetic Spin Structure in Exchange Biased Fe/FeSn2 Bilayers. Phys. Rev. B 2005, 72, 064440. [Google Scholar] [CrossRef]






| Milling Time (h) | Lattice Parameters (Å) | Crystallite Size (nm) | Phase Content (wt.%) | ||
|---|---|---|---|---|---|
| a | b | c | |||
| 0 | 5.0343 | - | 13.7430 | >50 | α-Fe2O3 (63.9) |
| 5.6229 | 4.8541 | 5.5374 | >100 | MoO2 (36.1) | |
| 2 | 5.0329 | - | 13.7286 | 38.9 | Mo: α-Fe2O3 (63.2) |
| 5.6199 | 4.8503 | 5.5372 | 72.5 | Fe: MoO2 (36.8) | |
| 4 | 5.0337 | - | 13.7403 | 29.2 | Mo: α-Fe2O3 (63.4) |
| 5.6212 | 4.8499 | 5.5408 | 63.3 | Fe: MoO2 (36.6) | |
| 8 | 5.0341 | - | 13.7413 | 23.1 | Mo: α-Fe2O3 (64.1) |
| 5.6188 | 4.8491 | 5.5447 | 56.5 | Fe: MoO2 (35.9) | |
| 12 | 5.0334 | - | 13.7537 | 17.7 | Mo: α-Fe2O3 (65.5) |
| 5.6180 | 4.8480 | 5.5457 | 49.9 | Fe: MoO2 (34.5) | |
| Statistical Errors | ±0.0007 | ±0.0007 | ±0.0007 | ±1.6 | ±1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolea, F.; Sorescu, M.; Diamandescu, L.; Iacob, N.; Tolea, M.; Kuncser, V. Unidirectional Magnetic Anisotropy in Molybdenum Dioxide–Hematite Mixed-Oxide Nanostructures. Nanomaterials 2022, 12, 938. https://doi.org/10.3390/nano12060938
Tolea F, Sorescu M, Diamandescu L, Iacob N, Tolea M, Kuncser V. Unidirectional Magnetic Anisotropy in Molybdenum Dioxide–Hematite Mixed-Oxide Nanostructures. Nanomaterials. 2022; 12(6):938. https://doi.org/10.3390/nano12060938
Chicago/Turabian StyleTolea, Felicia, Monica Sorescu, Lucian Diamandescu, Nicusor Iacob, Mugurel Tolea, and Victor Kuncser. 2022. "Unidirectional Magnetic Anisotropy in Molybdenum Dioxide–Hematite Mixed-Oxide Nanostructures" Nanomaterials 12, no. 6: 938. https://doi.org/10.3390/nano12060938