Luminescent Citrate-Functionalized Terbium-Substituted Carbonated Apatite Nanomaterials: Structural Aspects, Sensitized Luminescence, Cytocompatibility, and Cell Uptake Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Precipitation Technique
2.2. Physico-Chemical Characterizations
2.3. Luminescence Spectroscopy
2.4. Biological Tests
2.4.1. Cells Culture
2.4.2. Cell Proliferation Assays
2.4.3. Confocal Microscopy
2.4.4. Flow Cytometry
3. Results
3.1. Crystallographic, Compositional, Morphological and Spectroscopic Features of the Precipitates
3.2. Particle Size Distribution and ζ-Potential of Nanocolloids
3.3. Luminescence Properties in Solid State and in Aqueous Suspensions
3.4. Biological Tests
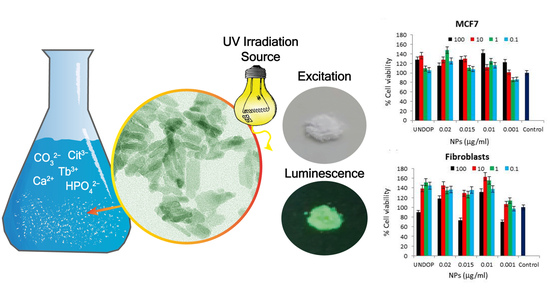
3.4.1. Cytocompatibility of the Particles
3.4.2. Flow Cytometry: Uptake and Intracellular Localization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sreenivasan, V.K.A.; Zvyagin, A.V.; Goldys, E.M. Luminescent nanoparticles and their applications in the life sciences. J. Phys. Condens. Matter 2013, 25, 194101–194124. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, J.-J. Quantum dots for fluorescent biosensing and bio-imaging applications. Analyst 2013, 138, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.C.; Lee, H.Y.; Chen, K.; Lim, T.S.; Wu, H.Y.; Lin, P.K.; Wei, P.K.; Tsao, P.H.; Chang, H.C.; Fann, W. Characterization and application of single fluorescent nanodiamonds as cellular biomarkers. Proc. Natl. Acad. Sci. USA 2007, 104, 727–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tan, W.B.; Zhang, Y.; Fan, X.; Wang, M. Luminescent nanomaterials for biological labelling. Nanotechnology 2005, 17, R1. [Google Scholar] [CrossRef]
- Oltolina, F.; Gregoletto, L.; Colangelo, D.; Gómez-Morales, J.; Delgado-López, J.M.; Prat, M. Monoclonal antibody-targeted fluorescein-5-isothiocyanate-labeled biomimetic nanoapatites: A promising fluorescent probe for imaging applications. Langmuir 2015, 31, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.S.H.; Han, Y.; Lu, X.; Wang, X.; Dai, H.; Li, S. Rare earth doped apatite nanomaterials for biological application. J. Nanomater. 2015, 2015, 705390. [Google Scholar] [CrossRef] [Green Version]
- Al-Kattan, A.; Santran, V.; Dufour, P.; Dexpert-Ghys, J.; Drouet, C. Novel contributions on luminescent apatite-based colloids intended for medical imaging. J. Biomater. Appl. 2014, 28, 697–707. [Google Scholar] [CrossRef] [Green Version]
- Al-Kattan, A.; Dufour, P.; Dexpert-Ghys, J.; Drouet, C. Preparation and physicochemical characteristics of luminescent apatite-based colloids. J. Phys. Chem. C 2010, 114, 2918–2924. [Google Scholar] [CrossRef]
- Liu, J.; Lécuyer, T.; Seguin, J.; Mignet, N.; Scherman, D.; Viana, B.; Richard, C. Imaging and therapeutic applications of persistent luminescence nanomaterials. Adv. Drug Deliv. Rev. 2019, 138, 193–210. [Google Scholar] [CrossRef]
- Rosticher, C.; Viana, B.; Maldiney, T.; Richard, C.; Chanéac, C. Persistent luminescence of Eu, Mn, Dy doped calcium phosphates for in-vivo optical imaging. J. Lumin. 2016, 170, 460–466. [Google Scholar] [CrossRef]
- Nikazar, S.; Sivasankarapillai, V.S.; Rahdar, A.; Gasmi, S.; Anumol, P.S.; Shanavas, M.S. Revisiting the cytotoxicity of quantum dots: An in-depth overview. Biophys. Rev. 2020, 12, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Morales, J.; Iafisco, M.; Delgado-López, J.M.; Sarda, S.; Drouet, C. Progress on the preparation of nanocrystalline apatites and surface characterization: Overview of fundamental and applied aspects. Prog. Cryst. Growth Charact. Mater. 2013, 59, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Iafisco, M.; Delgado-Lopez, J.M.; Varoni, E.M.; Tampieri, A.; Rimondini, L.; Gomez-Morales, J.; Prat, M. Cell surface receptor targeted biomimetic apatite nanocrystals for cancer therapy. Small 2013, 9, 3834–3844. [Google Scholar] [CrossRef] [PubMed]
- Drouet, C.; Al-Kattan, A.; Choimet, M.; Tourrette, A.; Santran, V.; Dexpert-Ghys, J.; Pipy, B.; Brouillet, F.; Tourbin, M. Biomimetic Apatite-Based Functional Nanoparticles as Promising Newcomers in Nanomedicine: Overview of 10 Years of Initiatory Research. Intern. Med. Prim. Healthc. 2015, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Grunenwald, A.; Keyser, C.; Sautereau, A.M.; Crubézy, E.; Ludes, B.; Drouet, C. Adsorption of DNA on biomimetic apatites: Toward the understanding of the role of bone and tooth mineral on the preservation of ancient DNA. Appl. Surf. Sci. 2014, 292, 867–875. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Ruiz, I.; Delgado-López, J.M.; Durán-Olivencia, M.A.; Iafisco, M.; Tampieri, A.; Colangelo, D.; Prat, M.; Gómez-Morales, J. pH-Responsive Delivery of Doxorubicin from Citrate–Apatite Nanocrystals with Tailored Carbonate Content. Langmuir 2013, 29, 8213–8221. [Google Scholar] [CrossRef]
- Iafisco, M.; Drouet, C.; Adamiano, A.; Pascaud, P.; Montesi, M.; Panseri, S.; Sarda, S.; Tampieri, A. Superparamagnetic iron-doped nanocrystalline apatite as a delivery system for doxorubicin. J. Mater. Chem. B 2015, 4, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.; Arribart, H.; Guille, M.M.G. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat. Mater. 2005, 4, 277–288. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef]
- López-Macipe, A.; Gómez-Morales, J.; Rodríguez-Clemente, R. Nanosized Hydroxyapatite Precipitation from Homogeneous Calcium/Citrate/Phosphate Solutions Using Microwave and Conventional Heating. Adv. Mater. 1998, 10, 49–53. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Rodríguez-Clemente, R.; Armas, B.; Combescure, C.; Berjoan, R.; Cubo, J.; Martínez, E.; García-Carmona, J.; Garelik, S.; Murtra, J.; et al. Controlled nucleation and growth of thin hydroxyapatite layers on titanium implants by using induction heating technique. Langmuir 2004, 20, 5174–5178. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, J.M.; Iafisco, M.; Rodríguez, I.; Tampieri, A.; Prat, M.; Gómez-Morales, J. Crystallization of bioinspired citrate-functionalized nanoapatite with tailored carbonate content. Acta Biomater. 2012, 8, 3491–3499. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, J.M.; Frison, R.; Cervellino, A.; Gómez-Morales, J.; Guagliardi, A.; Masciocchi, N. Crystal Size, Morphology, and Growth Mechanism in Bio-Inspired Apatite Nanocrystals. Adv. Funct. Mater. 2014, 24, 1090–1099. [Google Scholar] [CrossRef]
- Iafisco, M.; Ramírez-Rodríguez, G.B.; Sakhno, Y.; Tampieri, A.; Martra, G.; Gómez-Morales, J.; Delgado-López, J.M. The growth mechanism of apatite nanocrystals assisted by citrate: Relevance to bone biomineralization. CrystEngComm 2015, 17, 507–511. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Rawal, A.; Schmidt-Rohr, K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc. Natl. Acad. Sci. USA 2010, 107, 22425–22429. [Google Scholar] [CrossRef] [Green Version]
- Degli Esposti, L.; Adamiano, A.; Siliqi, D.; Giannini, C.; Iafisco, M. The effect of chemical structure of carboxylate molecules on hydroxyapatite nanoparticles. A structural and morphological study. Bioact. Mater. 2021, 6, 2360–2371. [Google Scholar] [CrossRef]
- Ivanchenko, P.; Delgado-López, J.M.; Iafisco, M.; Gómez-Morales, J.; Tampieri, A.; Martra, G.; Sakhno, Y. On the surface effects of citrates on nano-apatites: Evidence of a decreased hydrophilicity. Sci. Rep. 2017, 7, 8901. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Casado, F.J.; Iafisco, M.; Delgado-López, J.M.; Martínez-Benito, C.; Ruiz-Pérez, C.; Colangelo, D.; Oltolina, F.; Prat, M.; Gómez-Morales, J. Bioinspired citrate-apatite nanocrystals doped with divalent transition metal ions. Cryst. Growth Des. 2015, 16, 145–153. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Verdugo-Escamilla, C.; Fernández-Penas, R.; Parra-Milla, C.M.; Drouet, C.; Maube-Bosc, F.; Oltolina, F.; Prat, M.; Fernández-Sánchez, J.F. Luminescent biomimetic citrate-coated europium-doped carbonated apatite nanoparticles for use in bioimaging: Physico-chemistry and cytocompatibility. RSC Adv. 2018, 8, 2385–2397. [Google Scholar] [CrossRef] [Green Version]
- Jabalera, Y.; Oltolina, F.; Prat, M.; Jimenez-Lopez, C.; Fernández-Sánchez, J.F.; Choquesillo-Lazarte, D.; Gómez-Morales, J. Eu-Doped Citrate-Coated Carbonated Apatite Luminescent Nanoprobes for Drug Delivery. Nanomaterials 2020, 10, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Morales, J.; Verdugo-Escamilla, C.; Fernández-Penas, R.; Maria Parra-Milla, C.; Drouet, C.; Iafisco, M.; Oltolina, F.; Prat, M.; Fernández-Sánchez, J.F. Bioinspired crystallization, sensitized luminescence and cytocompatibility of citrate-functionalized Ca-substituted europium phosphate monohydrate nanophosphors. J. Colloid Interface Sci. 2019, 538, 174–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Morales, J.; Fernández-Penas, R.; Romero-Castillo, I.; Verdugo-Escamilla, C.; Choquesillo-Lazarte, D.; D’urso, A.; Prat, M.; Fernández-Sánchez, J.F. Crystallization, luminescence and cytocompatibility of hexagonal calcium doped terbium phosphate hydrate nanoparticles. Nanomaterials 2021, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Flores, Y.; Suárez-Quezada, M.; Rojas-Trigos, J.B.; Lartundo-Rojas, L.; Suárez, V.; Mantilla, A. Characterization of Tb-doped hydroxyapatite for biomedical applications: Optical properties and energy band gap determination. J. Mater. Sci. 2017, 52, 9990–10000. [Google Scholar] [CrossRef]
- Salvati, A.; Nelissen, I.; Haase, A.; Åberg, C.; Moya, S.; Jacobs, A.; Alnasser, F.; Bewersdorff, T.; Deville, S.; Luch, A.; et al. Quantitative measurement of nanoparticle uptake by flow cytometry illustrated by an interlaboratory comparison of the uptake of labelled polystyrene nanoparticles. NanoImpact 2018, 9, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Moreno, P.; Boulaiz, H.; Ortega-Vinuesa, J.L.; Peula-García, J.M.; Aránega, A. Novel drug delivery system based on docetaxel-loaded nanocapsules as a therapeutic strategy against breast cancer cells. Int. J. Mol. Sci. 2012, 13, 4906–4919. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Zhang, J.; Hu, X.; Yang, Z.; Guo, Y.; Wang, Y. A synergistic antibacterial effect between terbium ions and reduced graphene oxide in a poly(vinyl alcohol)-alginate hydrogel for treating infected chronic wounds. J. Mater. Chem. B 2019, 7, 538–547. [Google Scholar] [CrossRef]
- Liu, D.D.; Ge, K.; Jin, Y.; Sun, J.; Wang, S.X.; Yang, M.S.; Zhang, J.C. Terbium promotes adhesion and osteogenic differentiation of mesenchymal stem cells via activation of the Smad-dependent TGF-β/BMP signaling pathway. J. Biol. Inorg. Chem. 2014, 19, 879–891. [Google Scholar] [CrossRef]
- Romero-Castillo, I.; López-Ruiz, E.; Fernández-Sánchez, J.F.; Marchal, J.A.; Gómez-Morales, J. Self-Assembled Type I Collagen-Apatite Fibers with Varying Mineralization Extent and Luminescent Terbium Promote Osteogenic Differentiation of Mesenchymal Stem Cells. Macromol. Biosci. 2021, 21, 2000319. [Google Scholar] [CrossRef]
- Hirai, H.; Masui, T.; Imanaka, N.; Adachi, G. Characterization and thermal behavior of amorphous rare earth phosphates. J. Alloys Compd. 2004, 374, 84–88. [Google Scholar] [CrossRef]
- Vandecandelaere, N.; Rey, C.; Drouet, C. Biomimetic apatite-based biomaterials: On the critical impact of synthesis and post-synthesis parameters. J. Mater. Sci. Mater. Med. 2012, 23, 2593–2606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunenwald, A.; Keyser, C.; Sautereau, A.-M.; Crubézy, E.; Ludes, B.; Drouet, C. Revisiting carbonate quantification in apatite (bio)minerals: A validated FTIR methodology. J. Archaeol. Sci. 2014, 49, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts; Wiley: Hoboken, NJ, USA, 2001; p. 347. [Google Scholar]
- Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Gómez-Morales, J. Evolution of calcium phosphate precipitation in hanging drop vapor diffusion by in situ Raman microspectroscopy. CrystEngComm 2013, 15, 2206–2212. [Google Scholar] [CrossRef]
- Awonusi, A.; Morris, M.D.; Tecklenburg, M.M.J. Carbonate assignment and calibration in the Raman spectrum of apatite. Calcif. Tissue Int. 2007, 81, 46–52. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, K.P.F.; Lima, P.P.; Ferreira, R.A.S.; Carlos, L.D.; Bittar, E.M.; Matinaga, F.M.; Paniago, R.; Krambrock, K.; Moreira, R.L.; Dias, A. Influence of the Matrix on the Red Emission in Europium Self-Activated Orthoceramics. J. Phys. Chem. C 2015, 119, 17825–17835. [Google Scholar] [CrossRef]
- Siqueira, K.P.F.; Lima, P.P.; Ferreira, R.A.S.; Carlos, L.D.; Bittar, E.M.; Granado, E.; González, J.C.; Abelenda, A.; Moreira, R.L.; Dias, A. Lanthanide orthoantimonate light emitters: Structural, vibrational, and optical properties. Chem. Mater. 2014, 26, 6351–6360. [Google Scholar] [CrossRef]
- Medina-Velazquez, D.Y.; Caldiño, U.; Morales-Ramirez, A.; Reyes-Miranda, J.; Lopez, R.E.; Escudero, R.; Ruiz-Guerrero, R.; Morales Perez, M.F. Synthesis of luminescent terbium-thenoyltriflouroacetone MOF nanorods for green laser application. Opt. Mater. 2019, 87, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Richardson, F.S. Terbium(III) and Europium(III) Ions as Luminescent Probes and Stains for Biomolecular Systems. Chem. Rev. 1982, 82, 541–552. [Google Scholar] [CrossRef]
- Jiménez-Flores, Y.; Suárez-Quezada, M.; Rojas-Trigos, J.B.; Suárez, V.; Mantilla, A. Sol–gel synthesis of Tb-doped hydroxyapatite with high performance as photocatalyst for 2,4 dichlorophenoxyacetic acid mineralization. J. Chem. Technol. Biotechnol. 2017, 92, 1521–1530. [Google Scholar] [CrossRef]
- Gránásy, L.; Pusztai, T.; Tegze, G.; Warren, J.A.; Douglas, J.F. On the growth and form of spherulites. Phys. Rev. E 2015, 72, 011605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Jeong, K.J.; Kim, J.; Kang, S.W.; Kang, J.; Han, I.H.; Lee, I.W.; Oh, S.J.; Lee, J. Emission-tunable probes using terbium(III)-doped self-activated luminescent hydroxyapatite for in vitro bioimaging. J. Colloid Interface Sci. 2021, 581, 21–30. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. International Standard Organization: Geneva, Switzerland, 2009.
- Gustafsson, J.P. Visual MINTEQ 3.1, Freeware Chemical Equilibrium Model. Available online: https://vminteq.lwr.kth.se/ (accessed on 23 May 2021).
- Somasundaran, P. Zeta potential of apatite in aqueous solutions and its change during equilibration. J. Colloid Interface Sci. 1968, 27, 659–666. [Google Scholar] [CrossRef]
- López-Macipe, A.; Gómez-Morales, J.; Rodríguez-Clemente, R. The role of pH in the adsorption of citrate ions on hydroxyapatite. J. Colloid Interface Sci. 1998, 200, 114–120. [Google Scholar] [CrossRef]










| x, time mol/L Tb3+ | Ca (wt%) | P (wt%) | Tb (wt%) | (Ca + Tb)/P (mol) | H2Oads (wt%) | H2Ostr/b (wt%) | cit (wt%) | CO32− (wt%) |
|---|---|---|---|---|---|---|---|---|
| 0.001 M, 96 h | 31.8 ± 0.7 | 15.6 ± 0.4 | 1.59 ± 0.04 | 1.60 ± 0.01 | 3.2 ± 0.3 | 1.8 ± 0.2 | 1.9 ± 0.2 | 3.2 ± 0.3 |
| 0.001 M, 7 d | 31.1 ± 0.2 | 15.2 ± 0.2 | 1.50 ± 0.01 | 1.60 ± 0.01 | 3.4 ± 0.3 | 2.0 ± 0.2 | 1.7 ± 0.2 | 3.5 ± 0.3 |
| 0.005 M, 96 h | 26.8 ± 0.7 | 14.2 ± 0.4 | 7.33 ± 0.13 | 1.55 ± 0.01 | 3.5 ± 0.3 | 2.0 ± 0.2 | 1.3 ± 0.1 | 3.7 ± 0.4 |
| 0.005 M, 7 d | 25.9 ± 0.4 | 14.0 ± 0.2 | 7.51 ± 0.11 | 1.53 ± 0.01 | 3.9 ± 0.4 | 2.1 ± 0.2 | 1.3 ± 0.1 | 3.7 ± 0.4 |
| 0.010 M, 96 h | 21.5 ± 0.4 | 12.7 ± 0.2 | 12.80 ± 0.18 | 1.51 ± 0.01 | 6.0 ± 0.6 | 2.7 ± 0.3 | 1.8 ± 0.2 | 4.4 ± 0.4 |
| 0.010 M, 7 d | 22.2 ± 0.2 | 13.7 ± 0.1 | 14.20 ± 0.16 | 1.45 ± 0.01 | 4.5 ± 0.4 | 2.7 ± 0.3 | 1.5 ± 0.1 | 3.6 ± 0.4 |
| 0.015 M, 7 d | 17.0 ± 0.6 | 11.8 ± 0.4 | 18.72 ± 0.73 | 1.43 ± 0.01 | 7.6 ± 0.8 * | 3.2 ± 0.3 * | 2.2 ± 0.2 * | 2.6 ± 0.3 * |
| 0.020 M, 7 d | 14.5 ± 0.4 | 11.2 ± 0.4 | 21.93 ± 0.82 | 1.39 ± 0.01 | 10.9 ± 1.1 * | 4.4 ± 0.4 * | 2.6 ± 0.3 * | 2.3 ± 0.2 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Morales, J.; Fernández-Penas, R.; Acebedo-Martínez, F.J.; Romero-Castillo, I.; Verdugo-Escamilla, C.; Choquesillo-Lazarte, D.; Esposti, L.D.; Jiménez-Martínez, Y.; Fernández-Sánchez, J.F.; Iafisco, M.; et al. Luminescent Citrate-Functionalized Terbium-Substituted Carbonated Apatite Nanomaterials: Structural Aspects, Sensitized Luminescence, Cytocompatibility, and Cell Uptake Imaging. Nanomaterials 2022, 12, 1257. https://doi.org/10.3390/nano12081257
Gómez-Morales J, Fernández-Penas R, Acebedo-Martínez FJ, Romero-Castillo I, Verdugo-Escamilla C, Choquesillo-Lazarte D, Esposti LD, Jiménez-Martínez Y, Fernández-Sánchez JF, Iafisco M, et al. Luminescent Citrate-Functionalized Terbium-Substituted Carbonated Apatite Nanomaterials: Structural Aspects, Sensitized Luminescence, Cytocompatibility, and Cell Uptake Imaging. Nanomaterials. 2022; 12(8):1257. https://doi.org/10.3390/nano12081257
Chicago/Turabian StyleGómez-Morales, Jaime, Raquel Fernández-Penas, Francisco Javier Acebedo-Martínez, Ismael Romero-Castillo, Cristóbal Verdugo-Escamilla, Duane Choquesillo-Lazarte, Lorenzo Degli Esposti, Yaiza Jiménez-Martínez, Jorge Fernando Fernández-Sánchez, Michele Iafisco, and et al. 2022. "Luminescent Citrate-Functionalized Terbium-Substituted Carbonated Apatite Nanomaterials: Structural Aspects, Sensitized Luminescence, Cytocompatibility, and Cell Uptake Imaging" Nanomaterials 12, no. 8: 1257. https://doi.org/10.3390/nano12081257