Driven Engulfment of Janus Particles by Giant Vesicles in and out of Thermal Equilibrium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Colloids and Vesicles
2.2. Centrifugation
2.3. Microscopy and Tracking
3. Results and Discussion
3.1. Interaction between Janus Particles and GUVs Driven by Centrifugation
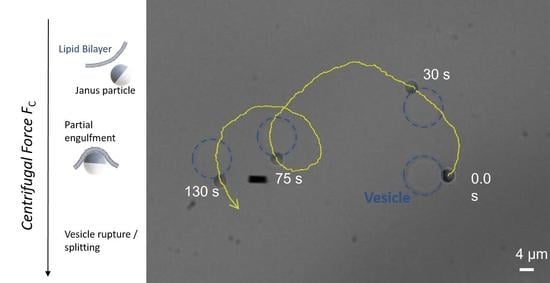
3.2. Partial Engulfment of Janus Particles by GUVs
3.3. Self-Propelled Janus Particles Partially Engulfed by GUVs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| DOTAP | 1,2-dioleoyl-3-trimethylammonium-propane |
| GUV | Giant Unilamellar Vesicle |
| MF | melamine formaldehyde |
| POPC | 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine |
| PS | polystyrene |
References
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natsume, Y.; Pravaz, O.; Yoshida, H.; Imai, M. Shape deformation of giant vesicles encapsulating charged colloidal particles. Soft Matter 2010, 6, 5359–5366. [Google Scholar] [CrossRef]
- Agudo-Canalejo, J.; Lipowsky, R. Critical particle sizes for the engulfment of nanoparticles by membranes and vesicles with bilayer asymmetry. ACS Nano 2015, 9, 3704–3720. [Google Scholar] [CrossRef] [PubMed]
- Frey, F.; Idema, T. More than just a barrier: Using physical models to couple membrane shape to cell function. Soft Matter 2021, 17, 3533–3549. [Google Scholar] [CrossRef] [PubMed]
- Petithory, T.; Pieuchot, L.; Josien, L.; Ponche, A.; Anselme, K.; Vonna, L. Size-Dependent Internalization Efficiency of Macrophages from Adsorbed Nanoparticle-Based Monolayers. Nanomaterials 2021, 11, 1963. [Google Scholar] [CrossRef] [PubMed]
- The Giant Vesicle Book; Dimova, R.; Marques, C.M. (Eds.) CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2019. [Google Scholar]
- Deserno, M.; Bickel, T. Wrapping of a spherical colloid by a fluid membrane. Europhys. Lett. 2003, 62, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, C.; Angelova, M.; Pouligny, B. Adhesion of Latex spheres to giant phospholipid vesicles: Statics and dynamics. J. Phys. II 1997, 7, 1651–1682. [Google Scholar] [CrossRef]
- Koltover, I.; Rädler, J.O.; Safinya, C.R. Membrane mediated attraction and ordered aggregation of colloidal particles bound to giant phospholipid vesicles. Phys. Rev. Lett. 1999, 82, 1991–1994. [Google Scholar] [CrossRef] [Green Version]
- Spanke, H.T.; Style, R.W.; François-Martin, C.; Feofilova, M.; Eisentraut, M.; Kress, H.; Agudo-Canalejo, J.; Dufresne, E.R. Wrapping of Microparticles by Floppy Lipid Vesicles. Phys. Rev. Lett. 2020, 125, 198102. [Google Scholar] [CrossRef]
- Ewins, E.J.; Han, K.; Bharti, B.; Robinson, T.; Velev, O.D.; Dimova, R. Controlled adhesion, membrane pinning and vesicle transport by Janus particles. Chem. Commun. 2022, 58, 3055–3058. [Google Scholar] [CrossRef]
- Vutukuri, H.R.; Hoore, M.; Abaurrea-Velasco, C.; van Buren, L.; Dutto, A.; Auth, T.; Fedosov, D.A.; Gompper, G.; Vermant, J. Active particles induce large shape deformations in giant lipid vesicles. Nature 2020, 586, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Azar, E.; Schroder, A.P.; Marques, C.M.; Stocco, A. Active colloids orbiting giant vesicles. Soft Matter 2021, 17, 4275–4281. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Gates, B.D.; Wolfe, D.B.; Paul, K.E.; Whitesides, G.M. Fabrication and Wetting Properties of Metallic Half-Shells with Submicron Diameters. Nano Lett. 2002, 2, 891–894. [Google Scholar] [CrossRef]
- Weinberger, A.; Tsai, F.C.; Koenderink, G.H.; Schmidt, T.F.; Itri, R.; Meier, W.; Schmatko, T.; Schröder, A.; Marques, C. Gel-assisted formation of giant unilamellar vesicles. Biophys. J. 2013, 105, 154–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocco, A.; Chollet, B.; Wang, X.; Blanc, C.; Nobili, M. Rotational diffusion of partially wetted colloids at fluid interfaces. J. Colloid Interface Sci. 2019, 542, 363–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigyou, K.; Nagai, K.H.; Hamada, T. Lateral Diffusion of a Submicrometer Particle on a Lipid Bilayer Membrane. Langmuir 2016, 32, 13771–13777. [Google Scholar] [CrossRef]
- Wang, X.; In, M.; Blanc, C.; Nobili, M.; Stocco, A. Enhanced active motion of Janus colloids at the water surface. Soft Matter 2015, 11, 7376–7384. [Google Scholar] [CrossRef] [Green Version]
- Mehr, F.N.; Grigoriev, D.; Puretskiy, N.; Böker, A. Mono-patchy zwitterionic microcolloids as building blocks for pH-controlled self-assembly. Soft Matter 2019, 15, 2430–2438. [Google Scholar] [CrossRef] [Green Version]
- Klasczyk, B.; Knecht, V.; Lipowsky, R.; Dimova, R. Interactions of alkali metal chlorides with phosphatidylcholine vesicles. Langmuir 2010, 26, 18951–18958. [Google Scholar] [CrossRef]
- Kollmitzer, B.; Heftberger, P.; Rappolt, M.; Pabst, G. Monolayer spontaneous curvature of raft-forming membrane lipids. Soft Matter 2013, 9, 10877–10884. [Google Scholar] [CrossRef] [Green Version]
- Goldmans, A.J.; Cox, R.G.; Brenner, H.; Neill, O. Slow viscous motion of a sphere parallel to a plane wall-1 Motion through a quiescent fluid. Chem. Eng. Sci. 1967, 22, 637. [Google Scholar] [CrossRef]
- Ketzetzi, S.; De Graaf, J.; Kraft, D.J. Diffusion-Based Height Analysis Reveals Robust Microswimmer-Wall Separation. Phys. Rev. Lett. 2020, 125, 238001. [Google Scholar] [CrossRef] [PubMed]
- Fragneto, G.; Charitat, T.; Daillant, J. Floating lipid bilayers: Models for physics and biology. Eur. Biophys. J. 2012, 41, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Cardoso Dos Santos, M.; Vézy, C.; Jaffiol, R. Nanoscale characterization of vesicle adhesion by normalized total internal reflection fluorescence microscopy. Biochim. Biophys. Acta Biomembr. 2016, 1858, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Howse, J.; Jones, R.; Ryan, A.; Gough, T.; Vafabakhsh, R.; Golestanian, R. Self-Motile Colloidal Particles: From Directed Propulsion to Random Walk. Phys. Rev. Lett. 2007, 99, 048102. [Google Scholar] [CrossRef] [Green Version]
- Ebbens, S.; Gregory, D.A.; Dunderdale, G.; Howse, J.R.; Ibrahim, Y.; Liverpool, T.B.; Golestanian, R. Electrokinetic effects in catalytic platinum-insulator Janus swimmers. Europhys. Lett. 2014, 106, 58003. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.; Poon, W. Ionic effects in self-propelled Pt-coated Janus swimmers. Soft Matter 2014, 10, 4016–4027. [Google Scholar] [CrossRef] [Green Version]
- Spagnolie, S.E.; Lauga, E. Hydrodynamics of self-propulsion near a boundary: Predictions and accuracy of far-field approximations. J. Fluid Mech. 2012, 700, 105–147. [Google Scholar] [CrossRef] [Green Version]
- Agudo-Canalejo, J.; Lipowsky, R. Uniform and Janus-like nanoparticles in contact with vesicles: Energy landscapes and curvature-induced forces. Soft Matter 2017, 13, 2155–2173. [Google Scholar] [CrossRef] [Green Version]
- Deserno, M. Elastic deformation of a fluid membrane upon colloid binding. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2004, 69, 031903. [Google Scholar] [CrossRef] [Green Version]
- Deserno, M. When do fluid membranes engulf sticky colloids? J. Phys. Condens. Matter 2004, 16, S2061–S2070. [Google Scholar] [CrossRef]
- Puu, G.; Gustafson, I. Planar lipid bilayers on solid supports from liposomes—Factors of importance for kinetics and stability. Biochim. Biophys. Acta Biomembr. 1997, 1327, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.H.; Min, Y.; Weirich, K.L.; Zeng, H.; Fygenson, D.; Israelachvili, J.N. Formation of supported bilayers on silica substrates. Langmuir 2009, 25, 6997–7005. [Google Scholar] [CrossRef] [PubMed]
- Baraban, L.; Tasinkevych, M.; Popescu, M.N.; Sanchez, S.; Dietrich, S.; Schmidt, O.G. Transport of cargo by catalytic Janus micro-motors. Soft Matter 2012, 8, 48. [Google Scholar] [CrossRef]








| MF–Pt Janus Colloids/GUVs | FC (pN) | Type of Events | |
|---|---|---|---|
| Adhesion/Engulfment | Membrane Rupture | ||
| POPC | 10 | ● (<10%) | |
| 100 | ● (30–40%) | ||
| 1000 | ● (<10%) | ● | |
| DOPC | 10 | ● (20–30%) | |
| 100 | ● (40–50%) | ||
| 1000 | ● (<10%) | ● | |
| SiO2–Pt Janus Colloids/GUVs | FC (pN) | Type of Events | |
|---|---|---|---|
| Adhesion/Engulfment | Membrane Rupture | ||
| POPC | 10 | ||
| 100 | ● (<10%) | ||
| 1000 | ● (<5%) | ● | |
| DOPC | 10 | ● (<2%) | ● |
| 100 | ● | ||
| 1000 | ● | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, V.; Marques, C.M.; Stocco, A. Driven Engulfment of Janus Particles by Giant Vesicles in and out of Thermal Equilibrium. Nanomaterials 2022, 12, 1434. https://doi.org/10.3390/nano12091434
Sharma V, Marques CM, Stocco A. Driven Engulfment of Janus Particles by Giant Vesicles in and out of Thermal Equilibrium. Nanomaterials. 2022; 12(9):1434. https://doi.org/10.3390/nano12091434
Chicago/Turabian StyleSharma, Vaibhav, Carlos M. Marques, and Antonio Stocco. 2022. "Driven Engulfment of Janus Particles by Giant Vesicles in and out of Thermal Equilibrium" Nanomaterials 12, no. 9: 1434. https://doi.org/10.3390/nano12091434