Superconducting Bio-Inspired Au-Nanowire-Based Neurons
Abstract
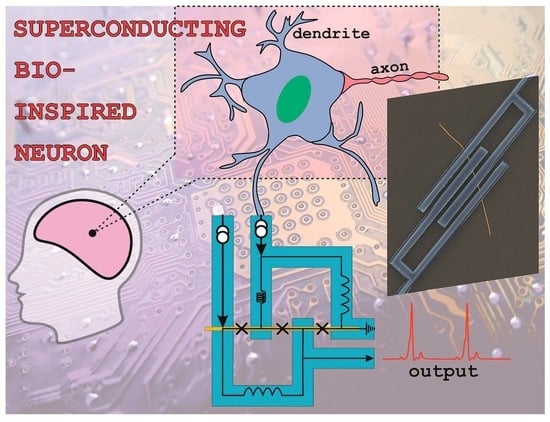
:1. Introduction
2. Results and Discussion
2.1. Preparation of Samples and Experimental Results
2.2. Bio-Inspired Neuron
- Regular mode shows the typical response of a neuron to external stimulation. A short input current pulse of a sufficient amplitude causes single spike, whereafter the system returns to a stable state, see Figure 4a. A long pulse leads to repeated overcoming of the firing threshold. Thus, a series of spikes is observed, Figure 4b. The interspike interval is determined by a neuron refractory period, which, in consequence, is related to the recovery of channels.
- Steady state mode (Figure 4c) is characterized by the weak damped output pulses. This is analog of the maintenance of constant internal concentrations of ions in the cell in response to an under threshold stimulation.
- Injury mode (Figure 4d) is characterized by the losses of spikes, or vice versa, the generation of “extra” spikes. This mimics the biophysical abnormality caused by different nervous diseases and neuron injuries.
- Bursting mode (Figure 4e) demonstrates the generation of a series of spikes in response to singe stimulating current pulse. Such behavior may be the result of the complex neuron interaction in the network. However, this can also be a consequence of internal processes in a neuron. In the last case, the reason is the after-depolarization (ADP), a membrane depolarisation at the last stages of repolarisation (circled “4” on the spike shown in Figure 4a) [59,60]. A slow sodium current appears at membrane voltage ∼−50…−70 mV and overcomes outward current, causing a membrane voltage to rise again. Such current is resistant to inactivation and may last for long times. The bursting pattern parameters—the spike sequence frequency and its length—are determined by the concentration of ion channels of different kinds, properties of these channels, and ionic concentrations in extracellular space. Though only relatively small cohort of neurons in vivo exhibits a bursting behavior [61,62,63], it plays an important role in synaptic plasticity [64,65], synchronization of big neuron groups [66], detection of frequency features of input stimuli [67], information encoding [68,69], and reliability of synaptic transmission [64,70], which may be crucial for processing of important stimuli [71].
- Figure 4f illustrates the bursting dynamics of biological neuron simulated in the frame of the fractional-order Izhikevich model [72,73] for comparison, see also Supplementary Materials.
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.D. Simulation of alcohol action upon a detailed Purkinje neuron model and a simpler surrogate model that runs >400 times faster. BMC Neurosci. 2015, 16, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Liu, H.; Uhlmann, G. On an inverse boundary problem arising in brain imaging. J. Differ. Equ. 2019, 267, 2471–2502. [Google Scholar] [CrossRef] [Green Version]
- Sætra, M.J.; Einevoll, G.T.; Halnes, G. An electrodiffusive, ion conserving Pinsky-Rinzel model with homeostatic mechanisms. PLoS Comput. Biol. 2020, 16, e1007661. [Google Scholar] [CrossRef] [PubMed]
- Sætra, M.J.; Einevoll, G.T.; Halnes, G. An electrodiffusive neuron-extracellular-glia model for exploring the genesis of slow potentials in the brain. PLoS Comput. Biol. 2021, 17, e1008143. [Google Scholar] [CrossRef]
- Chance, F. Lessons from a Dragon Fly’s Brain: Evolution Built a Small, Fast, Efficient Neural Network in a Dragonfly. Why Not Copy It for Missile Defense? IEEE Spectr. 2021, 58, 28–33. [Google Scholar] [CrossRef]
- Jürgensen, A.M.; Khalili, A.; Chicca, E.; Indiveri, G.; Nawrot, M.P. A neuromorphic model of olfactory processing and sparse coding in the Drosophila larva brain. Neuromorphic Comput. Eng. 2021, 1, 024008. [Google Scholar] [CrossRef]
- Ham, D.; Park, H.; Hwang, S.; Kim, K. Neuromorphic electronics based on copying and pasting the brain. Nat. Electron. 2021, 4, 635–644. [Google Scholar] [CrossRef]
- Toomey, E.; Segall, K.; Castellani, M.; Colangelo, M.; Lynch, N.; Berggren, K.K. Superconducting nanowire spiking element for neural networks. Nano Lett. 2020, 20, 8059–8066. [Google Scholar] [CrossRef]
- Markram, H.; Muller, E.; Ramaswamy, S.; Reimann, M.W.; Abdellah, M.; Sanchez, C.A.; Ailamaki, A.; Alonso-Nanclares, L.; Antille, N.; Arsever, S.; et al. Reconstruction and simulation of neocortical microcircuitry. Cell 2015, 163, 456–492. [Google Scholar] [CrossRef]
- Furber, S. Large-scale neuromorphic computing systems. J. Neural Eng. 2016, 13, 051001. [Google Scholar] [CrossRef] [PubMed]
- Sourikopoulos, I.; Hedayat, S.; Loyez, C.; Danneville, F.; Hoel, V.; Mercier, E.; Cappy, A. A 4-fJ/spike artificial neuron in 65 nm CMOS technology. Front. Neurosci. 2017, 11, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, F.A.; Carvalho, L.R.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.; Leite, R.E.; Filho, W.J.; Lent, R.; Herculano-Houzel, S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, W. Challenges and Applications of Emerging Nonvolatile Memory Devices. Electronics 2020, 9, 1029. [Google Scholar] [CrossRef]
- Chen, L.; Pam, M.E.; Li, S.; Ang, K.W. Ferroelectric memory based on two-dimensional materials for neuromorphic computing. Comput. Eng. 2022, 2, 022001. [Google Scholar] [CrossRef]
- Qiao, N.; Mostafa, H.; Corradi, F.; Osswald, M.; Stefanini, F.; Sumislawska, D.; Indiveri, G. A reconfigurable on-line learning spiking neuromorphic processor comprising 256 neurons and 128 K synapses. Front. Neurosci. 2015, 9, 141. [Google Scholar] [CrossRef] [Green Version]
- Holmes, D.S.; Ripple, A.L.; Manheimer, M.A. Energy-efficient superconducting computing—Power budgets and requirements. IEEE Trans. Appl. Supercond. 2013, 23, 1701610. [Google Scholar] [CrossRef]
- Tolpygo, S.K. Superconductor digital electronics: Scalability and energy efficiency issues. Low Temp. Phys. 2016, 42, 361–379. [Google Scholar] [CrossRef] [Green Version]
- Soloviev, I.I.; Klenov, N.V.; Bakurskiy, S.V.; Kupriyanov, M.Y.; Gudkov, A.L.; Sidorenko, A.S. Beyond Moore’s technologies: Operation principles of a superconductor alternative. Beilstein J. Nanotechnol. 2017, 8, 2689–2710. [Google Scholar] [CrossRef] [Green Version]
- Likharev, K.K. Dynamics of Josephson Junctions and Circuits; Routledge: London, UK, 2022. [Google Scholar]
- Barone, A.; Paterno, G. Physics and Applications of the Josephson Effect; Wiley Online Library: Hoboken, NJ, USA, 1982; Volume 1. [Google Scholar]
- Crotty, P.; Schult, D.; Segall, K. Josephson junction simulation of neurons. Phys. Rev. E 2010, 82, 011914. [Google Scholar] [CrossRef] [Green Version]
- Primavera, B.A.; Shainline, J.M. An active dendritic tree can mitigate fan-in limitations in superconducting neurons. Appl. Phys. Lett. 2021, 119, 242601. [Google Scholar] [CrossRef]
- Schneider, M.; Segall, K. Fan-out and fan-in properties of superconducting neuromorphic circuits. J. Appl. Phys. 2020, 128, 214903. [Google Scholar] [CrossRef]
- Soloviev, I.; Klenov, N.; Bakurskiy, S.; Bol’ginov, V.; Ryazanov, V.; Kupriyanov, M.Y.; Golubov, A.A. Josephson magnetic rotary valve. Appl. Phys. Lett. 2014, 105, 242601. [Google Scholar] [CrossRef] [Green Version]
- Bakurskiy, S.; Klenov, N.; Soloviev, I.; Kupriyanov, M.Y.; Golubov, A.A. Superconducting phase domains for memory applications. Appl. Phys. Lett. 2016, 108, 042602. [Google Scholar] [CrossRef] [Green Version]
- Bakurskiy, S.; Klenov, N.; Soloviev, I.; Pugach, N.; Kupriyanov, M.Y.; Golubov, A. Protected 0-π states in SIsFS junctions for Josephson memory and logic. Appl. Phys. Lett. 2018, 113, 082602. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.L.; Donnelly, C.A.; Russek, S.E.; Baek, B.; Pufall, M.R.; Hopkins, P.F.; Dresselhaus, P.D.; Benz, S.P.; Rippard, W.H. Ultralow power artificial synapses using nanotextured magnetic Josephson junctions. Sci. Adv. 2018, 4, e1701329. [Google Scholar] [CrossRef] [Green Version]
- Shafraniuk, S.E.; Nevirkovets, I.P.; Mukhanov, O.A. Modeling computer memory based on ferromagnetic/superconductor multilayers. Phys. Rev. Appl. 2019, 11, 064018. [Google Scholar] [CrossRef]
- Klenov, N.; Khaydukov, Y.; Bakurskiy, S.; Morari, R.; Soloviev, I.; Boian, V.; Keller, T.; Kupriyanov, M.; Sidorenko, A.; Keimer, B. Periodic Co/Nb pseudo spin valve for cryogenic memory. Beilstein J. Nanotechnol. 2019, 10, 833–839. [Google Scholar] [CrossRef]
- Likharev, K.K.; Semenov, V.K. RSFQ logic/memory family: A new Josephson-junction technology for sub-terahertz-clock-frequency digital systems. IEEE Trans. Appl. Supercond. 1991, 1, 3–28. [Google Scholar] [CrossRef]
- Tolpygo, S.K.; Bolkhovsky, V.; Rastogi, R.; Zarr, S.; Day, A.L.; Golden, E.; Weir, T.J.; Wynn, A.; Johnson, L.M. Advanced fabrication processes for superconductor electronics: Current status and new developments. IEEE Trans. Appl. Supercond. 2019, 29, 1–13. [Google Scholar] [CrossRef]
- Schegolev, A.E.; Klenov, N.V.; Soloviev, I.I.; Tereshonok, M.V. Adiabatic superconducting cells for ultra-low-power artificial neural networks. Beilstein J. Nanotechnol. 2016, 7, 1397–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segall, K.; LeGro, M.; Kaplan, S.; Svitelskiy, O.; Khadka, S.; Crotty, P.; Schult, D. Synchronization dynamics on the picosecond time scale in coupled Josephson junction neurons. Phys. Rev. E 2017, 95, 032220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soloviev, I.I.; Schegolev, A.E.; Klenov, N.V.; Bakurskiy, S.V.; Kupriyanov, M.Y.; Tereshonok, M.V.; Shadrin, A.V.; Stolyarov, V.S.; Golubov, A.A. Adiabatic superconducting artificial neural network: Basic cells. J. Appl. Phys. 2018, 124, 152113. [Google Scholar] [CrossRef] [Green Version]
- Schegolev, A.; Klenov, N.; Soloviev, I.; Tereshonok, M. Learning cell for superconducting neural networks. Supercond. Sci. Technol. 2020, 34, 015006. [Google Scholar] [CrossRef]
- Goteti, U.S.; Dynes, R.C. Superconducting neural networks with disordered Josephson junction array synaptic networks and leaky integrate-and-fire loop neurons. J. Appl. Phys. 2021, 129, 073901. [Google Scholar] [CrossRef]
- Ishida, K.; Byun, I.; Nagaoka, I.; Fukumitsu, K.; Tanaka, M.; Kawakami, S.; Tanimoto, T.; Ono, T.; Kim, J.; Inoue, K. Superconductor Computing for Neural Networks. IEEE Micro 2021, 41, 19–26. [Google Scholar] [CrossRef]
- Feldhoff, F.; Toepfer, H. Niobium Neuron: RSFQ Based Bio-Inspired Circuit. IEEE Trans. Appl. Supercond. 2021, 31, 1–5. [Google Scholar] [CrossRef]
- Semenov, V.K.; Golden, E.B.; Tolpygo, S.K. A new family of bioSFQ logic/memory cells. IEEE Trans. Appl. Supercond. 2021, 32, 1–5. [Google Scholar] [CrossRef]
- Soloviev, I.; Bakurskiy, S.; Ruzhickiy, V.; Klenov, N.; Kupriyanov, M.Y.; Golubov, A.; Skryabina, O.; Stolyarov, V. Miniaturization of Josephson Junctions for Digital Superconducting Circuits. Phys. Rev. Appl. 2021, 16, 044060. [Google Scholar] [CrossRef]
- Soloviev, I.; Ruzhickiy, V.; Bakurskiy, S.; Klenov, N.; Kupriyanov, M.Y.; Golubov, A.; Skryabina, O.; Stolyarov, V. Superconducting circuits without inductors based on bistable Josephson junctions. Phys. Rev. Appl. 2021, 16, 014052. [Google Scholar] [CrossRef]
- Faivre, T.; Golubev, D.; Pekola, J.P. Josephson junction based thermometer and its application in bolometry. J. Appl. Phys. 2014, 116, 094302. [Google Scholar] [CrossRef] [Green Version]
- Kirtley, J.R.; Paulius, L.; Rosenberg, A.J.; Palmstrom, J.C.; Holland, C.M.; Spanton, E.M.; Schiessl, D.; Jermain, C.L.; Gibbons, J.; Fung, Y.K.K.; et al. Scanning SQUID susceptometers with sub-micron spatial resolution. Rev. Sci. Instrum. 2016, 87, 093702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkler, A.; Segev, Y.; Myasoedov, Y.; Rappaport, M.L.; Ne’eman, L.; Vasyukov, D.; Zeldov, E.; Huber, M.E.; Martin, J.; Yacoby, A. Self-aligned nanoscale SQUID on a tip. Nano Lett. 2010, 10, 1046–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.; Winkelmann, C.B.; Courtois, H.; Gupta, A.K. Josephson coupling in the dissipative state of a thermally hysteretic μ-SQUID. Phys. Rev. B 2018, 98, 174514. [Google Scholar] [CrossRef] [Green Version]
- Dimov, B.; Balashov, D.; Khabipov, M.; Ortlepp, T.; Buchholz, F.I.; Zorin, A.; Niemeyer, J.; Uhlmann, F. Implementation of superconductive passive phase shifters in high-speed integrated RSFQ digital circuits. Supercond. Sci. Technol. 2008, 21, 045007. [Google Scholar] [CrossRef]
- Wiedenmann, J.; Bocquillon, E.; Deacon, R.S.; Hartinger, S.; Herrmann, O.; Klapwijk, T.M.; Maier, L.; Ames, C.; Brüne, C.; Gould, C.; et al. 4π-periodic Josephson supercurrent in HgTe-based topological Josephson junctions. Nat. Commun. 2016, 7, 10303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, F.; Hassler, F.; Platero, G. Dynamical detection of Majorana fermions in current-biased nanowires. Phys. Rev. B 2012, 86, 140503. [Google Scholar] [CrossRef] [Green Version]
- Kalenyuk, A.A.; Pagliero, A.; Borodianskyi, E.A.; Kordyuk, A.; Krasnov, V.M. Phase-Sensitive Evidence for the Sign-Reversal s ± Symmetry of the Order Parameter in an Iron-Pnictide Superconductor Using Nb/Ba1−x Nax Fe2 As2 Josephson Junctions. Phys. Rev. Lett. 2018, 120, 067001. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.H. Mathematical analysis of plasmonic resonance for 2-D photonic crystal. J. Differ. Equ. 2019, 266, 5095–5117. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Liu, H.; Zheng, G.H. Mathematical analysis of plasmon resonances for curved nanorods. J. Math. Pures Appl. 2019, 153, 248–280. [Google Scholar] [CrossRef]
- Skryabina, O.; Egorov, S.; Goncharova, A.; Klimenko, A.; Kozlov, S.; Ryazanov, V.; Bakurskiy, S.; Kupriyanov, M.Y.; Golubov, A.; Napolskii, K.; et al. Josephson coupling across a long single-crystalline Cu nanowire. Appl. Phys. Lett. 2017, 110, 222605. [Google Scholar] [CrossRef]
- Dubos, P.; Courtois, H.; Pannetier, B.; Wilhelm, F.; Zaikin, A.; Schön, G. Josephson critical current in a long mesoscopic SNS junction. Phys. Rev. B 2001, 63, 064502. [Google Scholar] [CrossRef] [Green Version]
- Courtois, H.; Meschke, M.; Peltonen, J.; Pekola, J.P. Origin of hysteresis in a proximity Josephson junction. Phys. Rev. Lett. 2008, 101, 067002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skryabina, O.; Bakurskiy, S.; Shishkin, A.; Klimenko, A.; Napolskii, K.; Klenov, N.; Soloviev, I.; Ryazanov, V.; Golubov, A.; Roditchev, D.; et al. Environment-induced overheating phenomena in Au-nanowire based Josephson junctions. Sci. Rep. 2021, 11, 15274. [Google Scholar] [CrossRef] [PubMed]
- Schindler, L.; Fourie, C.J. Application of Phase-Based Circuit Theory to RSFQ Logic Design. IEEE Trans. Appl. Supercond. 2022, 32, 1300512. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Katz, B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J. Physiol. 1949, 108, 37. [Google Scholar] [CrossRef]
- Azouz, R.; Jensen, M.S.; Yaari, Y. Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J. Physiol. 1996, 492, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Yue, C.; Remy, S.; Su, H.; Beck, H.; Yaari, Y. Proximal persistent Na+ channels drive spike afterdepolarizations and associated bursting in adult CA1 pyramidal cells. J. Neurosci. 2005, 25, 9704–9720. [Google Scholar] [CrossRef] [Green Version]
- Schwartzkroin, P.A. Characteristics of CA1 neurons recorded intracellularly in the hippocampalin vitro slice preparation. Brain Res. 1975, 85, 423–436. [Google Scholar] [CrossRef]
- Jensen, M.S.; Azouz, R.; Yaari, Y. Variant firing patterns in rat hippocampal pyramidal cells modulated by extracellular potassium. J. Neurophysiol. 1994, 71, 831–839. [Google Scholar] [CrossRef]
- Jensen, M.S.; Azouz, R.; Yaari, Y. Spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J. Physiol. 1996, 492, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.E. Bursts as a unit of neural information: Making unreliable synapses reliable. Trends Neurosci. 1997, 20, 38–43. [Google Scholar] [CrossRef]
- Selig, D.K.; Nicoll, R.A.; Malenka, R.C. Hippocampal long-term potentiation preserves the fidelity of postsynaptic responses to presynaptic bursts. J. Neurosci. 1999, 19, 1236–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, C.M.; McCormick, D.A. Chattering cells: Superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science 1996, 274, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Izhikevich, E.M.; Desai, N.S.; Walcott, E.C.; Hoppensteadt, F.C. Bursts as a unit of neural information: Selective communication via resonance. Trends Neurosci. 2003, 26, 161–167. [Google Scholar] [CrossRef]
- Cattaneo, A.; Maffei, L.; Morrone, C. Patterns in the discharge of simple and complex visual cortical cells. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1981, 212, 279–297. [Google Scholar]
- Otto, T.; Eichenbaum, H.; Wible, C.G.; Wiener, S.I. Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus 1991, 1, 181–192. [Google Scholar] [CrossRef]
- Williams, S.R.; Stuart, G.J. Mechanisms and consequences of action potential burst firing in rat neocortical pyramidal neurons. J. Physiol. 1999, 521, 467. [Google Scholar] [CrossRef]
- Fabian, J.M.; Wiederman, S.D. Spike bursting in a dragonfly target-detecting neuron. Sci. Rep. 2021, 11, 4005. [Google Scholar] [CrossRef]
- Izhikevich, E.M. Simple model of spiking neurons. IEEE Trans. Neural Netw. 2003, 14, 1569–1572. [Google Scholar] [CrossRef] [Green Version]
- Teka, W.W.; Upadhyay, R.K.; Mondal, A. Spiking and bursting patterns of fractional-order Izhikevich model. Commun. Nonlinear Sci. Numer. Simul. 2018, 56, 161–176. [Google Scholar] [CrossRef]
- Wang, B.; Ke, W.; Guang, J.; Chen, G.; Yin, L.; Deng, S.; He, Q.; Liu, Y.; He, T.; Zheng, R.; et al. Firing frequency maxima of fast-spiking neurons in human, monkey, and mouse neocortex. Front. Cell. Neurosci. 2016, 10, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, S.; Maunsell, J.H. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 2011, 9, e1000610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alekseichuk, I.; Turi, Z.; De Lara, G.A.; Antal, A.; Paulus, W. Spatial working memory in humans depends on theta and high gamma synchronization in the prefrontal cortex. Curr. Biol. 2016, 26, 1513–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connors, B.W.; Gutnick, M.J. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990, 13, 99–104. [Google Scholar] [CrossRef]
- Gruß, M.; Henrich, M.; König, P.; Hempelmann, G.; Vogel, W.; Scholz, A. Ethanol reduces excitability in a subgroup of primary sensory neurons by activation of BKCa channels. Eur. J. Neurosci. 2001, 14, 1246–1256. [Google Scholar] [CrossRef]
- Grafe, P.; Bostock, H.; Schneider, U. The effects of hyperglycaemic hypoxia on rectification in rat dorsal root axons. J. Physiol. 1994, 480, 297–307. [Google Scholar] [CrossRef]
- Oldham, K.; Spanier, J. The Fractional Calculus Theory and Applications of Differentiation and Integration to Arbitrary Order; Elsevier: Amsterdam, The Netherlands, 1974. [Google Scholar]
- Ionescu, C.; Lopes, A.; Copot, D.; Machado, J.T.; Bates, J.H. The role of fractional calculus in modeling biological phenomena: A review. Commun. Nonlinear Sci. Numer. Simul. 2017, 51, 141–159. [Google Scholar] [CrossRef]
- Caputo, M. Linear models of dissipation whose Q is almost frequency independent—II. Geophys. J. Int. 1967, 13, 529–539. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skryabina, O.V.; Schegolev, A.E.; Klenov, N.V.; Bakurskiy, S.V.; Shishkin, A.G.; Sotnichuk, S.V.; Napolskii, K.S.; Nazhestkin, I.A.; Soloviev, I.I.; Kupriyanov, M.Y.; et al. Superconducting Bio-Inspired Au-Nanowire-Based Neurons. Nanomaterials 2022, 12, 1671. https://doi.org/10.3390/nano12101671
Skryabina OV, Schegolev AE, Klenov NV, Bakurskiy SV, Shishkin AG, Sotnichuk SV, Napolskii KS, Nazhestkin IA, Soloviev II, Kupriyanov MY, et al. Superconducting Bio-Inspired Au-Nanowire-Based Neurons. Nanomaterials. 2022; 12(10):1671. https://doi.org/10.3390/nano12101671
Chicago/Turabian StyleSkryabina, Olga V., Andrey E. Schegolev, Nikolay V. Klenov, Sergey V. Bakurskiy, Andrey G. Shishkin, Stepan V. Sotnichuk, Kirill S. Napolskii, Ivan A. Nazhestkin, Igor I. Soloviev, Mikhail Yu. Kupriyanov, and et al. 2022. "Superconducting Bio-Inspired Au-Nanowire-Based Neurons" Nanomaterials 12, no. 10: 1671. https://doi.org/10.3390/nano12101671