A Strategy for Preparing Solid Polymer Electrolytes Containing In Situ Synthesized ZnO Nanoparticles with Excellent Electrochemical Performance
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
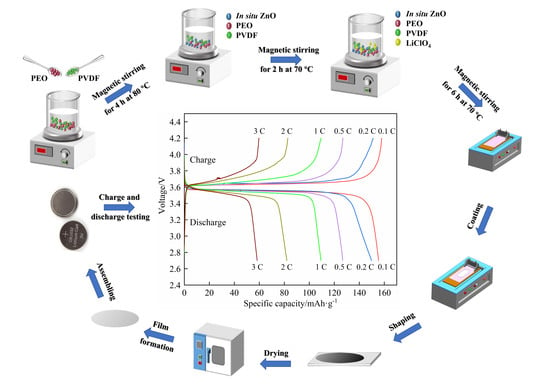
2.2. Preparation of In Situ ZnO and the SPEs
2.2.1. Preparation of In Situ ZnO
2.2.2. Preparation of the SPEs
2.3. Preparation of the SPEs with Directly-Added ZnO
2.4. Preparation of the Cathode
2.5. Material Characterization
2.6. Electrochemical Tests
3. Results and Discussion
3.1. Structural Characterization
3.2. Mechanical Properties
3.3. Electrochemical Tests
4. Conclusions
- (1)
- PEO–COO−-modified ZnO was successfully prepared by the three-step method. The SPEs were fabricated by blending PEO, PVDF, LiClO4, and in situ synthesized ZnO (0–5 wt.%) introduced with PEO-COO--modified ZnO as the intermediate.
- (2)
- The crystallinity of the SPEs reached the lowest value (2.04%) when adding a suitable content of in situ ZnO (3 wt.%). The SPE with 3 wt.% in situ ZnO also presented the best comprehensive mechanical properties in terms of the highest strength (2.736 MPa in yield strength, 4.749 MPa in tensile strength, and 2.855 MPa in breaking strength), plasticity (442.599% in elongation at break), and toughness.
- (3)
- The SPE with 3 wt.% in situ ZnO presented the best electrochemical performance, including the highest ion conductivity (1.378 × 10−4 S·cm−1 at 25 °C and 1.255 × 10−3 S·cm−1 at 60 °C) and migration number (0.858 at 60 °C), the widest electrochemical window (5.5 V at 60 °C), and the most outstanding rating capability (a capacity retention of 68.73%) and cycle stability (more than 1940 h).
- (4)
- Compared with directly-added ZnO, the in situ ZnO further improved the electrochemical performance of the SPEs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.; Hu, L.; Cheng, C.; Sun, K.; Guo, X.; Shao, Q.; Li, J.; Wang, N.; Guo, Z. Nano-TiNb2O7/carbon nanotubes composite anode for enhanced lithium-ion storage. Electrochim. Acta 2017, 260, 65–72. [Google Scholar] [CrossRef]
- Tian, J.; Shao, Q.; Dong, X.; Zheng, J.; Pan, D.; Zhang, X.; Cao, H.; Hao, L.; Liu, J.; Mai, X.; et al. Bio-template synthesized NiO/C hollow microspheres with enhanced Li-ion battery electrochemical performance. Electrochim. Acta 2017, 261, 236–245. [Google Scholar] [CrossRef]
- Utpalla, P.; Sharma, S.; Sudarshan, K.; Kumar, V.; Pujari, P. Free volume correlation with ac conductivity and thermo-mechanical properties of poly(ethylene oxide)-silica nanocomposites. Eur. Polym. J. 2019, 117, 10–18. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Huang, X.; Wu, J.; Wang, X.; Tian, Y.; Zhang, F.; Yang, M.; Xu, B.; Wu, B.; Liu, X.; Li, H. In Situ Synthesis of a Li6.4La3Zr1.4Ta0.6O12/Poly(vinylene carbonate) Hybrid Solid-State Electrolyte with Enhanced Ionic Conductivity and Stability. ACS Appl. Energy Mater. 2021, 4, 9368–9375. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Xu, T.; Li, L.; Gao, M.; Du, K.; Zhao, H.; Bai, Y. Fast ion conductor modified double-polymer (PVDF and PEO) matrix electrolyte for solid lithium-ion batteries. Solid State Ion. 2020, 355, 115419. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Shi, P.; Liu, Z.-Y.; Zhang, X.-Q.; Chen, X.; Yao, N.; Xie, J.; Jin, C.-B.; Zhan, Y.-X.; Ye, G.; Huang, J.-Q.; et al. Polar interaction of polymer host–solvent enables stable solid electrolyte interphase in composite lithium metal anodes. J. Energy Chem. 2021, 64, 172–178. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Li, W.; Xia, X.; Wang, X.; Gu, C.; Tu, J. A mono-comb poly(siloxane-g-ethylene oxide) electrospun fiber membrane for solid-state sodium ion batteries. Chem. Eng. J. 2021, 426, 131901. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2021, 64, 62–84. [Google Scholar] [CrossRef]
- Beshahwured, S.L.; Wu, Y.-S.; Truong, T.B.; Jose, R.; Yang, C.-C. A modified trilayer membrane for suppressing Li dendrite growth in all-solid-state lithium-metal batteries. Chem. Eng. J. 2021, 426, 131850. [Google Scholar] [CrossRef]
- Patil, V.S.; Vithya, K.; Premalatha, M.; Sundaresan, B. FTIR Studies on PMMA-LiNO3 Polymer Electrolyte. Macromol. Symp. 2019, 387, 1800177. [Google Scholar] [CrossRef]
- Koh, R.E.; Sun, C.C.; Yap, Y.L.; Cheang, P.L.; You, A.H. Investigation of Lithium Transference Number in PMMA Composite Polymer Electrolytes Using Monte Carlo (MC) Simulation and Recurrence Relation. J. Electrochem. Sci. Technol. 2021, 12, 217–224. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, M.; Peng, Y.; Guan, S. Organic ionic plastic crystal enhanced interface compatibility of PEO-based solid polymer electrolytes for lithium-metal batteries. Solid State Ion. 2021, 373, 115806. [Google Scholar] [CrossRef]
- Wang, X.; Hua, H.; Xie, X.; Zhang, P.; Zhao, J. Hydroxyl on the filler surface promotes Li+ conduction in PEO all-solid-state electrolyte. Solid State Ion. 2021, 372, 115768. [Google Scholar] [CrossRef]
- Khurana, S.; Chandra, A. Ionic liquid-based organic-inorganic hybrid electrolytes: Impact of in situ obtained and dispersed silica. J. Polym. Sci. Part B Polym. Phys. 2017, 56, 207–218. [Google Scholar] [CrossRef]
- Reddeppa, N.; Sharma, A.; Rao, V.N.; Chen, W. Preparation and characterization of pure and KBr doped polymer blend (PVC/PEO) electrolyte thin films. Microelectron. Eng. 2013, 112, 57–62. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, H.; Gao, Y.; Liang, X. High safety and long-life lithium batteries with low leakage and high wettability ceramic-polymer electrolyte. Ionics 2021, 27, 1113–1123. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Sengwa, R.J. Impact of PVDF/PEO blend composition on the β-phase crystallization and dielectric properties of silica nanoparticles incorporated polymer nanocomposites. J. Polym. Res. 2019, 26, 196. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Sengwa, R. Dielectric relaxation, Li-ion transport, electrochemical, and structural behaviour of PEO/PVDF/LiClO4/TiO2/PC-based plasticized nanocomposite solid polymer electrolyte films. Compos. Commun. 2019, 17, 182–191. [Google Scholar] [CrossRef]
- Banitaba, S.N.; Semnani, D.; Heydari-Soureshjani, E.; Rezaei, B.; Ensafi, A.A. Effect of titanium dioxide and zinc oxide fillers on morphology, electrochemical and mechanical properties of the PEO-based nanofibers, applicable as an electrolyte for lithium-ion batteries. Mater. Res. Express 2019, 6, 850d6. [Google Scholar] [CrossRef]
- Patil, S.U.; Yawale, S.S.; Yawale, S.P. Conductivity study of PEO-LiClO4 polymer electrolyte doped with ZnO nanocomposite ceramic filler. Bull. Mater. Sci. 2014, 37, 1403–1409. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Li, W. Evolution in electrochemical performance of the solid blend polymer electrolyte (PEO/PVDF) with the content of ZnO nanofiller. Colloids Surf. A Physicochem. Eng. Asp. 2021, 632, 127773. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, J.; Xu, M.; Xia, Q.; Liu, J.; Zhao, S.; Chen, L.; Pan, A.; Ivey, D.G.; Wei, W. Cross-linked branching nanohybrid polymer electrolyte with monodispersed TiO2 nanoparticles for high performance lithium-ion batteries. J. Power Sources 2016, 317, 103–111. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, T.; Chu, X.; Su, H.; Wang, Z.; Chen, N.; Gu, B.; Zhang, H.; Deng, W.; Zhang, H.; et al. Strong Lewis Acid–Base and Weak Hydrogen Bond Synergistically Enhancing Ionic Conductivity of Poly(ethylene oxide)@SiO2 Electrolytes for a High Rate Capability Li-Metal Battery. ACS Appl. Mater. Interfaces 2020, 12, 10341–10349. [Google Scholar] [CrossRef]
- Hou, G.M.; Zhang, M.Q.; Huang, Y.F.; Ruan, W.H. TiO2/PEO composite incorporated with in situ synthesized hyper-branched poly (amine-ester) and its application for polymer electrolyte. RSC Adv. 2016, 6, 83406–83411. [Google Scholar] [CrossRef]
- Tan, X.; Wu, Y.; Tang, W.; Song, S.; Yao, J.; Wen, Z.; Lu, L.; Savilov, S.V.; Hu, N.; Molenda, J. Preparation of Nanocomposite Polymer Electrolyte via In Situ Synthesis of SiO2 Nanoparticles in PEO. Nanomaterials 2020, 10, 157. [Google Scholar] [CrossRef] [Green Version]
- Chaurasia, S.K.; Chandra, A. Organic-inorganic hybrid electrolytes by in-situ dispersion of silica nanospheres in polymer matrix. Solid State Ion. 2017, 307, 35–43. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Wang, X.; Zhou, A.; Yang, Z. Applying multi-scale silica-like three-dimensional networks in a PEO matrix via in situ crosslinking for high-performance solid composite electrolytes. Mater. Chem. Front. 2021, 5, 7767–7777. [Google Scholar] [CrossRef]
- Chen, W.; Xiong, X.; Zeng, R.; Jiang, L.; Chen, Z.; Xiao, Z.; Qie, L.; Yu, F.; Huang, Y. Enhancing the Interfacial Ionic Transport via in Situ 3D Composite Polymer Electrolytes for Solid-State Lithium Batteries. ACS Appl. Energy Mater. 2020, 3, 7200–7207. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Liao, C.; Gong, W.; Yang, M.; Li, R.K.Y.; Tjong, S.C.; Lu, Z. Enhanced electrochemical performance of solid PEO/LiClO4 electrolytes with a 3D porous Li6.28La3Zr2Al0.24O12 network. Compos. Sci. Technol. 2019, 184, 107863. [Google Scholar] [CrossRef]
- Sengwa, R.; Dhatarwal, P. Predominantly chain segmental relaxation dependent ionic conductivity of multiphase semicrystalline PVDF/PEO/LiClO4 solid polymer electrolytes. Electrochim. Acta 2020, 338, 135890. [Google Scholar] [CrossRef]
- Prasanth, R.; Shubha, N.; Hng, H.H.; Srinivasan, M. Effect of poly(ethylene oxide) on ionic conductivity and electrochemical properties of poly(vinylidenefluoride) based polymer gel electrolytes prepared by electrospinning for lithium ion batteries. J. Power Sources 2014, 245, 283–291. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Wang, J.; Huang, D.; Chen, L.; Yang, G. Hybrid gel polymer electrolyte fabricated by electrospinning technology for polymer lithium-ion battery. Eur. Polym. J. 2015, 67, 365–372. [Google Scholar] [CrossRef]
- Przyluski, J.; Siekierski, M.; Wieczorek, W. Effective medium theory in studies of conductivity of composite polymeric electrolytes. Electrochim. Acta 1995, 40, 2101–2108. [Google Scholar] [CrossRef]
- Wang, Y. The Crystallization Structure and Tension Deformation Behavior of Nanoparticle-Polymer Composite. Master’s Thesis, Chongqing University, Chongqing, China, 2015. [Google Scholar]
- Lei, R.; Yang, Y.; Yu, C.; Xu, Y.; Li, Y.; Li, J. A facile preparation of PEO–LiClO4–fumed SiO2 composite solid-state electrolyte with improved electrochemical performance for lithium-metal batteries. Sustain. Energy Fuels 2021, 5, 1538–1547. [Google Scholar] [CrossRef]
- Rathika, R.; Padmaraj, O.; Suthanthiraraj, S.A. Electrical conductivity and dielectric relaxation behaviour of PEO/PVdF-based solid polymer blend electrolytes for zinc battery applications. Ionics 2017, 24, 243–255. [Google Scholar] [CrossRef]
- Polu, A.R.; Rhee, H.-W. Effect of Organic–Inorganic Hybrid Nanoparticles (POSS–PEG(n = 4)) on Thermal, Mechanical, and Electrical Properties of PEO-Based Solid Polymer Electrolytes. Adv. Polym. Technol. 2015, 36, 145–151. [Google Scholar] [CrossRef]
- Nie, K.; Wang, X.; Qiu, J.; Wang, Y.; Yang, Q.; Xu, J.; Yu, X.; Li, H.; Huang, X.; Chen, L. Increasing Poly(ethylene oxide) Stability to 4.5 V by Surface Coating of the Cathode. ACS Energy Lett. 2020, 5, 826–832. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, M.; Gao, X.; Bao, D.; Sun, Q.; Holmes, N.; Duan, H.; Mukherjee, S.; Adair, K.; Zhao, C.; et al. Determining the limiting factor of the electrochemical stability window for PEO-based solid polymer electrolytes: Main chain or terminal –OH group? Energy Environ. Sci. 2020, 13, 1318–1325. [Google Scholar] [CrossRef]
- Lang, J.; Song, J.; Qi, L.; Luo, Y.; Luo, X.; Wu, H. Uniform Lithium Deposition Induced by Polyacrylonitrile Submicron Fiber Array for Stable Lithium Metal Anode. ACS Appl. Mater. Interfaces 2017, 9, 10360–10365. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, M.; Arca, E.; Ha, Y.; Stetson, C.; Teeter, G.; Han, S.-D.; Stradins, P. Enhanced Interfacial Stability of Si Anodes for Li-Ion Batteries via Surface SiO2 Coating. ACS Appl. Energy Mater. 2020, 3, 8842–8849. [Google Scholar] [CrossRef]
- Hao, X.; Zhao, Q.; Su, S.; Zhang, S.; Ma, J.; Shen, L.; Yu, Q.; Liu, Y.; Kang, F.; He, Y. Constructing Multifunctional Interphase between Li1.4Al0.4Ti1.6(PO4)3 and Li Metal by Magnetron Sputtering for Highly Stable Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2019, 9, 1901604. [Google Scholar] [CrossRef]









| Sample | PEO | PVDF | 0 wt.% ZnO [26] | 1 wt.% ZnO | 2 wt.% ZnO | 3 wt.% ZnO | 4 wt.% ZnO | 5 wt.% ZnO |
|---|---|---|---|---|---|---|---|---|
| (°C) | - | - | 48.16 | 38.71 | 37.38 | 34.24 | 40.05 | 41.63 |
| (J·g−1) | - | - | 15.20 | 7.96 | 6.83 | 4.35 | 9.83 | 10.45 |
| (%) | 80 | 50–60 | 7.11 | 3.72 | 3.20 | 2.04 | 4.60 | 4.89 |
| Sample | 0 wt.% ZnO [26] | 1 wt.% ZnO | 2 wt.% ZnO | 3 wt.% ZnO | 4 wt.% ZnO | 5 wt.% ZnO |
|---|---|---|---|---|---|---|
| Yield strength/MPa | 0.978 | 1.413 | 1.800 | 2.376 | 1.274 | 1.204 |
| Tensile strength/MPa | 1.281 | 2.815 | 3.589 | 4.749 | 2.547 | 2.004 |
| Breaking strength/MPa | 0.766 | 1.690 | 2.155 | 2.855 | 1.588 | 1.202 |
| Elongation at break/% | 66.167 | 291.904 | 305.396 | 442.599 | 180.297 | 46.479 |
| Sample | 0 wt.% ZnO [26] | 1 wt.% ZnO | 2 wt.% ZnO | 3 wt.% ZnO | 4 wt.% ZnO | 5 wt.% ZnO |
|---|---|---|---|---|---|---|
| d (cm) | 0.0086 | 0.0091 | 0.0119 | 0.0113 | 0.0127 | 0.0138 |
| (Ω) | 30.760 | 6.013 | 7.042 | 4.595 | 9.960 | 13.440 |
| (10−4 S·cm−1) | 1.426 | 7.721 | 8.622 | 12.547 | 6.506 | 5.239 |
| Sample | 25 °C | 40 °C | 60 °C | 80 °C |
|---|---|---|---|---|
| 0 wt.% ZnO [26] | 2.247 × 10−5 | 4.124 × 10−5 | 1.426 × 10−4 | 3.447 × 10−4 |
| 1 wt.% ZnO | 7.476 × 10−5 | 1.621 × 10−4 | 7.721 × 10−4 | 1.427 × 10−3 |
| 2 wt.% ZnO | 8.131 × 10−5 | 2.107 × 10−4 | 8.622 × 10−4 | 1.543 × 10−3 |
| 3 wt.% ZnO | 1.378 × 10−4 | 5.213 × 10−4 | 1.255 × 10−3 | 2.199 × 10−3 |
| 4 wt.% ZnO | 7.092 × 10−5 | 1.486 × 10−4 | 6.506 × 10−4 | 1.310 × 10−3 |
| 5 wt.% ZnO | 5.445 × 10−5 | 1.143 × 10−4 | 5.239 × 10−4 | 1.061 × 10−3 |
| Sample | 0 wt.% ZnO [26] | 1 wt.% ZnO | 2 wt.% ZnO | 3 wt.% ZnO | 4 wt.% ZnO | 5 wt.% ZnO |
|---|---|---|---|---|---|---|
| (mV) | 10 | 10 | 10 | 10 | 10 | 10 |
| (μA) | 2.541 | 1.775 | 4.156 | 1.437 | 1.966 | 1.595 |
| (μA) | 1.707 | 1.449 | 3.503 | 1.239 | 1.567 | 1.245 |
| (Ω) | 460.6 | 225.7 | 94.79 | 76.73 | 277.5 | 299.3 |
| (Ω) | 472.9 | 221.3 | 85.66 | 47.33 | 275.6 | 285.2 |
| 0.645 | 0.810 | 0.835 | 0.858 | 0.788 | 0.771 |
| Refs. | Materials | Ion Conductivity (S·cm−1) | Migration Number | Electrochemical Window (V) | Cycle Performance | Rate Performance |
|---|---|---|---|---|---|---|
| This work | PEO/PVDF/LiClO4 /in situ ZnO | 1.378 × 10−4 (25 °C) 1.255 × 10−3 (60 °C) | 0.858 | 5.50 | 1940 h at 0.02 mA·cm−2 and 98.9%/50 cycles at 1 C (60 °C) | 106.8 mAh·g−1 at 1 C (60 °C) |
| Our previous work [26] | PEO/PVDF/LiClO4 /ZnO | 7.17 × 10−5 (25 °C) 3.145 × 10−4 (60 °C) | 0.768 | 5.25 | 1000 h at 0.02 mA·cm−2 and 95.3%/50 cycles at 1 C (60 °C) | 93.4 mAh·g−1 at 1 C (60 °C) |
| [28] | PEO/LiClO4/SiO2 | 1.1 × 10−4(30 °C) | 0.367 | 4.8 | 400 h at 0.1 mA·cm−2 and 70%/100 cycles at 0.2 C (90 °C) | 90 mAh·g−1 at 2 C (90 °C) |
| [30] | PEO/LiClO4/SiO2 | 1.1 × 10−4(30 °C) | — | 5.0 | 700 h at 0.01 mA·cm−2 and 70%/100 cycles at 0.2 C (55 °C) | 81 mAh·g−1 at 0.2 C (55 °C) |
| [31] | PEO/LiTf/SiO2 | 1.24 × 10−6 (30 °C) | 0.39 | 4.8 | — | — |
| [32] | PEO/LiTFSI/PEA/ γ-GPS/mPEG/SiO2 | 2.52 × 10−4 (40 °C) | 0.2046 | 5.1 | 800 h at 0.1 mA·cm−2 and 89.3%/50 cycles at 0.5 C (50 °C) | 117 mAh·g−1 at 0.5 C (50 °C) |
| [33] | PEO/LiTFSI/ TiO2 | 3.95 × 10−4 (60 °C) | — | 5.0 | 600 h at 0.1 mA·cm−2 and 85.1%/100 cycles at 100 mA·g−1 (60 °C) | 86 mAh·g−1 at 100 mA·g−1 (60 °C) |
| [34] | PEO/LiClO4/Li6.28La3Zr2Al0.24O12 | 2.25 × 10−5 (30 °C) | 0.263 | 5.5 | 100 h at 0.3 mA·cm−2 and 94.8%/100 cycles at 1 C (60 °C) | 136 mAh·g−1 at 1 C (60 °C) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Li, J.; Li, W. A Strategy for Preparing Solid Polymer Electrolytes Containing In Situ Synthesized ZnO Nanoparticles with Excellent Electrochemical Performance. Nanomaterials 2022, 12, 2680. https://doi.org/10.3390/nano12152680
Xu Y, Li J, Li W. A Strategy for Preparing Solid Polymer Electrolytes Containing In Situ Synthesized ZnO Nanoparticles with Excellent Electrochemical Performance. Nanomaterials. 2022; 12(15):2680. https://doi.org/10.3390/nano12152680
Chicago/Turabian StyleXu, Yinsi, Jun Li, and Wanggen Li. 2022. "A Strategy for Preparing Solid Polymer Electrolytes Containing In Situ Synthesized ZnO Nanoparticles with Excellent Electrochemical Performance" Nanomaterials 12, no. 15: 2680. https://doi.org/10.3390/nano12152680