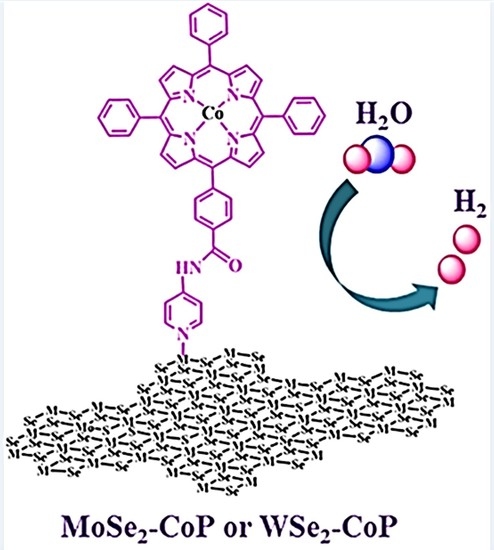
Molybdenum Diselenide and Tungsten Diselenide Interfacing Cobalt-Porphyrin for Electrocatalytic Hydrogen Evolution in Alkaline and Acidic Media
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, X.; Tang, C.S.; Zheng, Z.; Gao, J.; Wu, J.; Zhang, H.; Chhowalla, M.; Chen, W.; Wee, A.T.S. Recent developments in 2D transition metal dichalcogenides: Phase transition and applications of the (quasi-)metallic phases. Chem. Soc. Rev. 2021, 50, 10087–10115. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, G.H.; Cho, J.; Amani, M.; Mastandrea, J.P.; Groschner, C.K.; Lien, D.-H.; Zhao, Y.; Ager, J.W.; Scott, M.C.; et al. Synthetic WSe2 monolayers with high photoluminescence quantum yield. Sci. Adv. 2019, 5, eaau4728. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Zhang, T.; Yao, J.; Zhang, Y.; Xu, J.; Yang, G. Flexible, transparent and ultra-broadband photodetector based on large-area WSe2 film for wearable devices. Nanotechnology 2016, 27, 225501. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.R.; Enyashin, A.N.; Houben, L.; Bar-Ziv, R.; Bar-Sada, M. Ni–WSe2 nanostructures as efficient catalysts for electrochemical hydrogen evolution reaction (HER) in acidic and alkaline media. J. Mater. Chem. A 2020, 8, 1403–1416. [Google Scholar] [CrossRef]
- Kagkoura, A.; Arenal, R.; Tagmatarchis, N. Controlled chemical functionalization toward 3D-2D carbon nanohorn-MoS2 heterostructures with enhanced electrocatalytic activity for protons reduction. Adv. Funct. Mater. 2021, 31, 2105287. [Google Scholar] [CrossRef]
- Kagkoura, A.; Pelaez-Fernandez, M.; Arenal, R.; Tagmatarchis, N. Sulfur-doped graphene/transition metal dichalcogenide heterostructured hybrids with electrocatalytic activity toward the hydrogen evolution reaction. Nanoscale Adv. 2019, 1, 1489–1496. [Google Scholar] [CrossRef] [Green Version]
- Kagkoura, A.; Tzanidis, I.; Dracopoulos, V.; Tagmatarchis, N.; Tasis, D. Template synthesis of defect-rich MoS2-based assemblies as electrocatalytic platforms for hydrogen evolution reaction. Chem. Commun. 2019, 55, 2078–2081. [Google Scholar] [CrossRef] [Green Version]
- Kagkoura, A.; Canton-Vitoria, R.; Vallan, L.; Hernandez-Ferrer, J.; Benito, A.M.; Maser, W.K.; Arenal, R.; Tagmatarchis, N. Bottom-Up Synthesized MoS2 Interfacing Polymer Carbon Nanodots with Electrocatalytic Activity for Hydrogen Evolution. Chem. Eur. J. 2020, 26, 6635–6642. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, J.; Wang, Z.; Zhang, W. 2D MoSe2/CoP intercalated nanosheets for efficient electrocatalytic hydrogen production. Int. J. Hydrog. Energy. 2020, 45, 9246–19256. [Google Scholar] [CrossRef]
- Vishnoi, P.; Pramoda, K.; Gupta, U.; Chhetri, M.; Balakrishna, R.G.; Rao, C.N.R. Covalently Linked Heterostructures of Phosphorene with MoS2/MoSe2 and Their Remarkable Hydrogen Evolution Reaction Activity. ACS Appl. Mater. Interfaces 2019, 11, 27780–27787. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.W.; Hwang, H.; Yoon, S.I.; Lee, Z.; Shin, H.S. Vertically oriented MoS2/WS2 heterostructures on reduced graphene oxide sheets as electrocatalysts for hydrogen evolution reaction. Mater. Chem. Front. 2021, 5, 3396–3403. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Shaikh, S.F.; Ubaidullah, M.; Ghanem, A.M.; Mane, R.S. Self-grown one-dimensional nickel sulfo-selenide nanostructured electrocatalysts for water splitting reactions. Int. J. Hydrog. Energy. 2020, 45, 15904–15914. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Patil, S.A.; Truong, L.; Arbab, A.A.; Jeong, S.H.; Chun, S.-H.; Jung, J.; Kim, H.S. Engineering MoSe2/WS2 Hybrids to Replace the Scarce Platinum Electrode for Hydrogen Evolution Reactions and Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 5061–5072. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Cai, Q.; Hong, W.; Li, J.; Liu, W. Edge-Riched MoSe2/MoO2 Hybrid Electrocatalyst for Efficient Hydrogen Evolution Reaction. Small 2018, 14, 1703798. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Lunardon, M.; Bortoli, M.; Mosconi, D.; Girardi, L.; Orian, L.; Agnoli, S.; Granozzi, G. Tuning on and off chemical- and photo-activity of exfoliated MoSe2 nanosheets through morphologically selective “soft” covalent functionalization with porphyrins. J. Mater. Chem. A 2020, 8, 11019–11030. [Google Scholar] [CrossRef]
- Kwon, I.S.; Kwak, I.H.; Abbas, H.G.; Seo, H.W.; Seo, J.; Park, K.; Park, J.; Kang, H.S. Two dimensional MoS2 meets porphyrins via intercalation to enhance the electrocatalytic activity toward hydrogen evolution. Nanoscale 2019, 11, 3780–3785. [Google Scholar] [CrossRef]
- Mahmood, N.; Yao, Y.; Zhang, J.-W.; Pan, L.; Zhang, X.; Zou, J.-J. Electrocatalysts for hydrogen evolution in alkaline electrolytes: Mechanisms, challenges, and prospective solutions. Adv. Sci. 2018, 5, 1700464. [Google Scholar] [CrossRef]
- Kagkoura, A.; Stangel, C.; Arenal, R.; Tagmatachis, N. Molybdenum diselenide–manganese porphyrin bifunctional electrocatalyst for the hydrogen evolution reaction and selective hydrogen peroxide production. J. Phys. Chem. C 2022, 126, 14850–14858. [Google Scholar] [CrossRef]
- Iglesias, D.; Ippolito, S.; Ciesielski, A.; Samorì, P. Simultaneous non-covalent bi-functionalization of 1T-MoS2 ruled by electrostatic interactions: Towards multi-responsive materials. Chem. Commun. 2020, 56, 6878–6881. [Google Scholar] [CrossRef]
- Canton-Vitoria, R.; Stangel, C.; Tagmatarchis, N. Electrostatic association of ammonium-functionalized layered-transition-metal dichalcogenides with an anionic porphyrin. ACS Appl. Mater. Interfaces 2018, 10, 23476–23480. [Google Scholar] [CrossRef]
- Chen, X.; Denninger, P.; Stimpel-Lindner, T.; Spiecker, E.; Duesberg, G.S.; Backes, C.; Knirsch, K.C.; Hirsch, A. Defect engineering of two-dimensional molybdenum disulfide. Chem. Eur. J. 2020, 26, 6535–6544. [Google Scholar] [CrossRef] [PubMed]
- Canton-Vitoria, R.; Scharl, T.; Stergiou, A.; Cadranel, R.; Arenal, R.; Guldi, D.M.; Tagmatarchis, N. Ping-pong energy transfer in covalently linked porphyrin-MoS2 architectures. Angew. Chem. Int. Ed. 2020, 59, 3976–3981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Bartlam, C.; Lloret, V.; Badlyan, N.M.; Wolff, S.; Gillen, R.; Stimpel-Lindner, T.; Maultzsch, J.; Duesberg, G.S.; Knirsch, K.C.; et al. Covalent bisfunctionalization of two-dimensional molybdenum disulfide. Angew. Chem. Int. Ed. 2021, 60, 13484–13492. [Google Scholar] [CrossRef]
- Vera-Hidalgo, M.; Giovanelli, E.; Navío, C.; Pérez, E.M. Mild covalent functionalization of transition metal dichalcogenides with maleimides: A “click” reaction for 2H-MoS2 and WS2. J. Am. Chem. Soc. 2019, 141, 3767–3771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gali, S.M.; Wang, C.; Pershin, A.; Slassi, A.; Beljonne, D.; Samorì, P. Molecular functionalization of chemically active defects in WSe2 for enhanced opto-electronics. Adv. Funct. Mater. 2020, 30, 2005045. [Google Scholar] [CrossRef]
- Sideri, I.; Arenal, R.; Tagmatarchis, N. Covalently functionalized MoS2 with dithiolenes. ACS Mater. Lett. 2020, 2, 832–837. [Google Scholar] [CrossRef]
- Knirsch, K.C.; Berner, N.C.; Nerl, H.C.; Cucinotta, C.S.; Gholamvand, Z.; McEvoy, N.; Wang, Z.; Abramovic, I.; . Vecera, P.; Halik, M.; et al. Basal-plane functionalization of chemically exfoliated molybdenum disulfide by diazonium salts. ACS Nano 2015, 9, 6018–6030. [Google Scholar] [CrossRef] [PubMed]
- Yan, E.X.; Caban-Acevedo, M.; Papadantonakis, K.M.; Brunschwig, B.S.; Lewis, N.S. 1T′-MoS2, Reductant-activated, high-coverage, covalent functionalization of 1T′-MoS2. ACS Mater. Lett. 2020, 2, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Voiry, D.; Goswami, A.; Kappera, R.; de Carvalho Castro Silva, C.; Kaplan, D.; Fujita, T.; Chen, M.; Asefa, T.; Chhowalla, M. Covalent functionalization of monolayered transition metal dichalcogenides by phase engineering. Nat. Chem. 2015, 7, 45–49. [Google Scholar] [CrossRef]
- Presolski, S.; Wang, L.; Loo, A.H.; Ambrosi, A.; Lazar, P.; Ranc, V.; Otyepka, M.; Zboril, R.; Tomanec, O.; Ugolotti, J.; et al. Functional nanosheet synthons by covalent modification of transition-metal dichalcogenides. Chem. Mater. 2017, 29, 2066–2073. [Google Scholar] [CrossRef]
- Chen, X.; McAteer, D.; McGuinness, C.; Godwin, I.; Coleman, J.N.; McDonald, A.R. Tuning the photo-electrochemical performance of RuII-sensitized two-dimensional MoS2. Chem. Eur. J. 2018, 24, 351–355. [Google Scholar] [CrossRef]
- Li, W.; Chen, D.; Xia, F.; Tan, J.Z.Y.; Song, J.; Song, W.-G.; Caruso, R.A. Flowerlike WSe2 and WS2 microspheres: One-pot synthesis, formation mechanism and application in heavy metal ion sequestration. Chem. Commun. 2016, 52, 4481–4484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeanguillaume, C.; Colliex, C. Spectrum-image: The next step in EELS digital acquisition and processing. Ultramicroscopy 1989, 28, 252–257. [Google Scholar] [CrossRef]
- Arenal, R.; de la Pena, F.; Stephan, O.; Walls, M.; Loiseau, A.; Colliex, C. Extending the analysis of EELS spectrum-imaging data, from elemental to bond mapping in complex nanostructures. Ultramicroscopy 2008, 109, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-Q.; Chen, J.-S.; Niu, H.-L.; Mao, C.-J.; Son, J.-M. Synthesis of ultrathin WSe2 nanosheets and their high-performance catalysis for conversion of amines to imines. Nanoscale 2018, 10, 20266–20271. [Google Scholar] [CrossRef] [PubMed]
- del Corro, E.; Botello-Méndez, A.; Gillet, Y.; Elias, A.L.; Terrones, H.; Feng, S.; Fantini, C.; Rhodes, D.; Pradhan, N.; Balicas, L.; et al. Atypical exciton–phonon interactions in WS2 and WSe2 monolayers revealed by resonance Raman spectroscopy. Nano Lett. 2016, 16, 2363–2368. [Google Scholar] [CrossRef]
- Tan, S.M.; Sofer, Z.; Luxa, J.; Pumera, M. Aromatic-exfoliated transition metal dichalcogenides: Implications for inherent electrochemistry and hydrogen evolution. ACS Catal. 2016, 6, 4594–4607. [Google Scholar] [CrossRef]
- Tonndorf, P.; Schmidt, R.; Bottger, P.; Zhang, X.; Borner, J.; Liebig, A.; Albrecht, M.; Kloc, C.; Gordan, O.; Zahn, D.R.T.S.; et al. Photoluminescence emission and Raman response of monolayer MoS2, MoSe2, and WSe2. Opt. Express 2013, 21, 4908–4916. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J.; Wu, M.; Jian, W.; Xue, H.; Ng, T.-W.; Lee, C.-S.; Xu, J. Synthesis of 1T-MoSe2 ultrathin nanosheets with an expanded interlayer spacing of 1.17 nm for efficient hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 14949–14953. [Google Scholar] [CrossRef]
- Ambrosi, A.; Sofer, Z.; Pumera, M. 2H → 1T phase transition and hydrogen evolution activity of MoS2, MoSe2, WS2 and WSe2 strongly depends on the MX2 composition. Chem. Commun. 2015, 51, 8450–8453. [Google Scholar] [CrossRef]
- Sokolikova, M.S.; Sherrell, P.C.; Palczynski, P.; Bemmer, V.L.; Mattevi, C. Direct solution-phase synthesis of 1T′ WSe2 nanosheets. Nat. Commun. 2019, 10, 712. [Google Scholar]
- McCrum, I.T.; Koper, M.T.M. The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum. Nat. Energy 2020, 5, 891–899. [Google Scholar] [CrossRef]
- Dubouis, N.; Grimaud, A. The hydrogen evolution reaction: From material to interfacial descriptors. Chem. Sci. 2019, 10, 9165–9181. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kagkoura, A.; Stangel, C.; Arenal, R.; Tagmatarchis, N. Molybdenum Diselenide and Tungsten Diselenide Interfacing Cobalt-Porphyrin for Electrocatalytic Hydrogen Evolution in Alkaline and Acidic Media. Nanomaterials 2023, 13, 35. https://doi.org/10.3390/nano13010035
Kagkoura A, Stangel C, Arenal R, Tagmatarchis N. Molybdenum Diselenide and Tungsten Diselenide Interfacing Cobalt-Porphyrin for Electrocatalytic Hydrogen Evolution in Alkaline and Acidic Media. Nanomaterials. 2023; 13(1):35. https://doi.org/10.3390/nano13010035
Chicago/Turabian StyleKagkoura, Antonia, Christina Stangel, Raul Arenal, and Nikos Tagmatarchis. 2023. "Molybdenum Diselenide and Tungsten Diselenide Interfacing Cobalt-Porphyrin for Electrocatalytic Hydrogen Evolution in Alkaline and Acidic Media" Nanomaterials 13, no. 1: 35. https://doi.org/10.3390/nano13010035