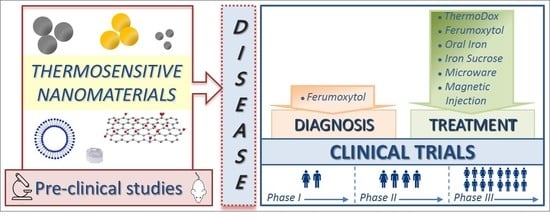
Clinical Trials of Thermosensitive Nanomaterials: An Overview
Abstract
:1. Introduction
1.1. Inorganic Nanoparticles
1.2. Carbon-Based Nanomaterials
1.3. Liposomal Formulations
2. Clinical Trials and Marketed Thermosensitive Nanomedicines
2.1. Thermosensitive Nanomaterials for Disease Diagnosis
2.1.1. Ferumoxytol as a Current Imaging Test Enhancer
2.1.2. Magnetic Nanoparticles for Sperm Sorting
2.2. Thermosensitive Nanomaterials for Disease Treatment
2.2.1. Iron Deficiency Anemia in Patients with Chronic Kidney Disease
2.2.2. Cardiovascular Diseases
2.2.3. Diabetes
2.2.4. Cancer
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peppas, N.A.; Clegg, J.R. The challenge to improve the response of biomaterials to the physiological environment. Regen. Biomater. 2016, 3, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Sahle, F.F.; Gulfam, M.; Lowe, T.L. Design strategies for physical-stimuli-responsive programmable nanotherapeutics. Drug Discov. Today 2018, 23, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.M.; Veeranarayanan, S.; Maekawa, T.; Kumar, S.D. External stimulus responsive inorganic nanomaterials for cancer theranostics. Adv. Drug Deliv. 2018, in press. [Google Scholar] [CrossRef]
- Sánchez-Moreno, P.; de Vicente, J.; Nardecchia, S.; Marchal, J.A.; Boulaiz, H. Thermo-sensitive nanomaterials: Recents advance in synthesis and biomedical applications. Nanomaterials 2018, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Datta, N.R.; Gómez Ordóñez, S.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Huang, P.; Fan, W.; Wang, Z.; Liu, Y.; Wang, S.; Zhang, G.; Hu, J.; Liu, W.; Niu, G.; et al. Tri-stimuli-responsive biodegradable theranostics for mild hyperthermia enhanced chemotherapy. Biomaterials 2017, 126, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Guisasola, E.; Baeza, A.; Asín, L.; de la Fuente, J.M.; Vallet-Regí, M. Heating at the nanoscale through drug-delivery devices: Fabrication and synergic effects in cancer treatment with nanoparticles. Small Methods 2018, 2, 1800007. [Google Scholar] [CrossRef]
- Hayashi, K.; Ono, K.; Suzuki, H.; Sawada, M.; Moriya, M.; Sakamoto, W.; Yogo, T. High-frequency, magnetic-field-responsive drug release from magnetic nanoparticle/organic hybrid based on hyperthermic effect. ACS Appl. Mater. Interfaces 2010, 2, 1903–1911. [Google Scholar] [CrossRef]
- Cherukula, K.; Manickavasagam Lekshmi, K.M.; Uthaman, S.; Cho, K.; Cho, C.-S.; Park, I.-K. Multifunctional inorganic nanoparticles: Recent progress in thermal therapy and imaging. Nanomaterials 2016, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Thorek, D.L.J.; Chen, A.K.; Czupryna, J.; Tsourkas, A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann. Biomed. Eng. 2006, 34, 23–38. [Google Scholar] [CrossRef] [PubMed]
- D’Acunto, M. Detection of intracellular gold nanoparticles: An overview. Materials 2018, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef]
- Jennings, L.E.; Long, N.J. ‘Two is better than one’-probes for dual-modality molecular imaging. Chem. Commun. 2009, 3511–3524. [Google Scholar] [CrossRef]
- Misri, R.; Meier, D.; Yung, A.C.; Kozlowski, P.; Häfeli, U.O. Development and evaluation of a dual-modality (MRI/SPECT) molecular imaging bioprobe. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1007–1016. [Google Scholar] [CrossRef]
- Zhao, M.; Beauregard, D.A.; Louizou, L.; Davletov, B.; Brindle, K.M. Non-invasive detection of apoptosis using magnetic resonance imaging and a targeted contrast agent. Nat. Med. 2001, 7, 1241–1244. [Google Scholar] [CrossRef]
- Seo, H.I.; Cheon, Y.A.; Chung, B.G. Graphene and thermo-responsive polymeric nanocomposites for therapeutic applications. Biomed. Eng. Lett. 2016, 6, 10–15. [Google Scholar] [CrossRef]
- Kneidl, B.; Peller, M.; Winter, G.; Hossann, M. Thermosensitive liposomal drug delivery systems: State of the art review. Int. J. Nanomed. 2014, 9, 4387–4398. [Google Scholar] [CrossRef]
- Ye, F.; Qin, J.; Toprak, M.S. Multifunctional core-shell nanoparticles: Superparamagnetic, mesoporous, and thermosensitive. J. Nanopart. Res. 2011, 13, 6157–6167. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef] [PubMed]
- Brazel, C.S. Magnetothermally-responsive nanomaterials: Combining magnetic nanostructures and thermally-sensitive polymers for triggered drug release. Pharm. Res. 2009, 26, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Delgado, A.V.; Schneider, E.K.; Checa Fernández, B.L.; Iglesias, G.R. Magnetic nanoparticles coated with a thermosensitive polymer with hyperthermia properties. Polymers 2018, 10, 10. [Google Scholar] [CrossRef]
- Shen, L.; Li, B.; Qiao, Y. Fe3O4 nanoparticles in targeted drug/gene delivery systems. Materials 2018, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Stafford, S.; Serrano Garcia, R.; Gun’ko, Y.K. Multimodal magnetic-plasmonic nanoparticles for biomedical applications. Appl. Sci. 2018, 8, 97. [Google Scholar] [CrossRef]
- Lee, N.; Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for multimodal synergistic cancer therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for in vivo imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, M. Multifunctional magnetic nanoparticles for medical imaging applications. J. Mater. Chem. 2009, 19, 6258–6266. [Google Scholar] [CrossRef] [PubMed]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef]
- Justin, C.; Philip, S.A.; Samrot, A.V. Synthesis and characterization of superparamagnetic iron-oxide nanoparticles (SPIONs) and utilization of SPIONs in X-ray imaging. Appl. Nanosci. 2017, 7, 463–475. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G.; Sudame, A.; Luthra, T.; Saini, K.; Maity, D. Functionalized hydrophilic superparamagnetic iron oxide nanoparticles for magnetic fluid hyperthermia application in liver cancer treatment. ACS Omega 2018, 3, 3991–4005. [Google Scholar] [CrossRef] [PubMed]
- Boulaiz, H.; Alvarez, P.J.; Ramirez, A.; Marchal, J.A.; Prados, J.; Rodríguez-Serrano, F.; Perán, M.; Melguizo, C.; Aranega, A. Nanomedicine: Application areas and development prospects. Int. J. Mol. Sci. 2011, 12, 3303–3321. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Review of some interesting surface Plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics 2007, 2, 107–118. [Google Scholar] [CrossRef]
- Moreira, A.F.; Rodrigues, C.F.; Reis, C.A.; Costa, E.C.; Correia, I.J. Gold-core silica shell nanoparticles application in imaging and therapy: A review. Microporous Mesoporous Mater. 2018, 270, 168–179. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G.; et al. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew. Chem. 2013, 52, 13958–13964. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent progress in cancer thermal therapy using gold nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic phothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef]
- Li, S.; Peng, Z.; Han, X.; Leblanc, R.M. Interactions between grapheme oxide and biomolecules from surface chemistry and spectroscopy. Recent Prog. Colloid Surf. Chem. Biol. Appl. ACS Symp. Ser. 2015, 1215, 43–64. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene nanomaterials: Synthesis, biocompatibility, and citotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Zhang, S.; Winyard, P.G. Developing the next generation of gaphene-based platforms for cancer therapeutics: The potential role of reactive oxygen species. Redox Biol. 2018, 15, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Koning, G.A.; Li, L.; ten Hagen, T.L.M. Thermosensitive liposomes for the delivery of cancer therapeutics. Ther. Deliv. 2010, 1, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. Stimuli-responsive smart liposomes in cancer targeting. Curr. Drug Targets 2018, 19, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An update review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Affram, K.; Udofot, O.; Singh, M.; Krishnan, S.; Reams, R.; Rosenberg, J.; Agyare, E. Smart thermosensitive liposomes for effective solid tumor therapy and in vivo imaging. PLoS ONE 2017, 12, e0185116. [Google Scholar] [CrossRef]
- Dicheva, B.M.; ten Hagen, T.L.M.; Seynhaeve, A.L.B.; Amin, M.; Eggermont, A.M.M.; Koning, G.A. Enhanced specificity and drug delivery in tumors by cRGD-anchiring thermosensitive liposomes. Pharm. Res. 2015, 32, 3862–3876. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.; Anyarambhatla, G.; Kong, G.; Dewhirst, M.W. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res. 2000, 60, 1197–1201. [Google Scholar] [PubMed]
- Dou, Y.; Hynynen, K.; Allen, C. To heat or not to heat: Challenges with clinical translation of thermosensitive liposomes. J. Control. Release 2017, 249, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Lindner, L.H.; Eichhorn, M.E.; Eibl, H.; Teichert, N.; Schmitt-Sody, M.; Issels, R.D.; Dellian, M. Novel Temperature-Sensitive Liposomes with Prolonged Circulation Time. Clin. Cancer Res. 2004, 10, 2168–2178. [Google Scholar] [CrossRef] [PubMed]
- Limmer, S.; Hahn, J.; Schmidt, R.; Wachholz, K.; Zengerle, A.; Lechner, K.; Eibl, H.; Issels, R.D.; Hossann, M.; Lindner, L.H. Gemcitabine treatment of rat soft tissue sarcoma with phosphatidyldiglycerol-based thermosensitive liposomes. Pharm. Res. 2014, 31, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.N.; Zheng, J.; Foltz, R.; Weersink, R.; Chaudary, N.; Jaffray, D.A.; Allen, C. Heat-activated thermosensitive liposomal cisplatin (HTLC) results in effective growth delay of cervical carcinoma in mice. J. Control. Release 2014, 178, 69–78. [Google Scholar] [CrossRef] [PubMed]
- May, J.P.; Ernsting, M.J.; Undzys, E.; Li, S.D. Thermosensitive liposomes for the delivery of gemcitabine and oxaliplatin to tumors. Mol. Pharm. 2013, 10, 4499–4508. [Google Scholar] [CrossRef] [PubMed]
- Sneider, A.; VanDyke, D.; Paliwal, S.; Rai, P. Remotely triggered nano-theranostics for cancer applications. Nanotheranostics 2017, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Hwang, K.; Lu, Y. Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics 2016, 6, 1336–1352. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Han, S.; Li, H.; Zhao, F.; Su, X.; Cao, X.; Zhang, Z. Multi-functional liposomes showing radiofrequency-triggered release and magnetic resonance imaging for tumor multi-mechanism therapy. Nanoscale 2015, 7, 5411–5426. [Google Scholar] [CrossRef] [PubMed]
- Garello, F.; Terreno, E. Sonosensitive MRI nanosystems as cancer theranostics: A recent update. Front. Chem. 2018, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Rizzitelli, S.; Giustetto, P.; Faletto, D.; Delli Castelli, D.; Aime, S.; Terreno, E. The release of Doxorubicin from liposomes monitored by MRI and triggered by a combination of US stimuli led to a complete tumor regression in a breast cancer mouse model. J. Control. Release 2016, 230, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zheng, M.; Luo, Z.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Ma, Y.; Cai, L. NIR-driven smart theranostic nanomedicine for on-demand drug release and synergistic antitumour therapy. Sci. Rep. 2015, 5, 14258. [Google Scholar] [CrossRef]
- Meng, L.; Deng, Z.; Niu, L.; Li, F.; Yan, F.; Wu, J.; Cai, F.; Zheng, H. A disposable microfluidic device for controlled drug release from thermal-sensitive liposomes by high intensity focused ultrasound. Theranostics 2015, 5, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Banno, B.; Ickenstein, L.M.; Chiu, G.N.C.; Bally, M.B.; Thewalt, J.; Brief, E.; Wasan, E.K. The functional roles of poly(ethylene glycol)-lipid and lysolipid in the drug retention and release from lysolipid-containing thermosensitive liposomes in vitro and in vivo. J. Pharm. Sci. 2010, 99, 2295–2308. [Google Scholar] [CrossRef] [PubMed]
- Kokuryo, D.; Nakashima, S.; Ozaki, F.; Yuba, E.; Chuang, K.-H.; Aoshima, S.; Ishizaka, Y.; Saga, T.; Kono, K.; Aoki, I. Evaluation of thermo-triggered drug release in intramuscular-transplanted tumors using thermosensitive polymer-modified liposomes and MRI. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 229–238. [Google Scholar] [CrossRef]
- Shemesh, C.S.; Hardy, C.W.; Yu, D.S.; Fernandez, B.; Zhang, H. Indocyanine green loaded liposome nanocarriers for photodynamic therapy using human triple negative breast cancer cells. Photodiagn. Photodyn. Ther. 2014, 11, 193–203. [Google Scholar] [CrossRef]
- Sun, Q.; You, Q.; Wang, J.; Liu, L.; Wang, Y.; Song, Y.; Cheng, Y.; Wang, S.; Tan, F.; Li, N. Theranostic nanoplatform: Triple-modal imaging-guided synergistic cancer therapy based on liposome-conjugated mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 1963–1975. [Google Scholar] [CrossRef]
- Andrews, J. The Role of Eicosanoids in the Cardiovascular Actions of Inhaled Nanoparticles (ECOARM); ClinicalTrials.gov Identifier: NCT03659864; Centre for Cardiovascular Science: Edinburgh, UK, 2018. [Google Scholar]
- Wang, Y.X.; Idee, J.M. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef]
- Bashir, M.R.; Batti, L.; Marin, D.; Nelson, R.C. Emerging applications for ferumoxytol as a contrast agent in MRI. J. Magn. Reson. Imaging 2015, 41, 884–898. [Google Scholar] [CrossRef]
- Hamilton, B.E.; Nesbit, G.M.; Dosa, E.; Gahramanov, S.; Rooney, B.; Nesbit, E.G.; Raines, J.; Neuwelt, E.A. Comparative analysis of ferumoxytol and gadoteridol enhancement using T1- and T2-weighted MRI in neuroimaging. Am. J. Roentgenol. 2011, 197, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Toth, G.B.; Varallyay, C.G.; Horvath, A.; Bashir, M.R.; Choyke, P.L.; Daldrup-Link, H.E.; Dosa, E.; Finn, J.P.; Gahramanov, S.; Harisinghani, M.; et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. 2017, 92, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E. Ferumoxytol- and Gadolinium-Labeled MRI in Measuring Tumors before or after Treatment in Patients with Primary or Metastatic Brain; ClinicalTrials.gov Identifier: NCT00659126; OHSU Knight Cancer Institute: Portland, OR, USA, 2008. [Google Scholar]
- Fuller, C. Ferumoxytol-Iron Oxide Nanoparticle Magnetic Resonance Dynamic Contrast Enhanced MRI; ClinicalTrials.gov Identifier: NCT01895829; University of Texas MD Anderson Cancer Center: Houston, TX, USA, 2013. [Google Scholar]
- Ipsen. MM-398 (Nanoliposomal Irinotecan, NaI-IRI) to Determine Tumor Drug Levels and to Evaluate the Feasibility of Ferumoxytol Magnetic Resonance Imaging to Measure Tumor Associated Macrophages and to Predict Patient Response to Treatment; ClinicalTrials.gov Identifier: NCT01770353; Mayo Clinic and HonorHealth: Scottsdale, AZ, USA, 2013. [Google Scholar]
- Guimaraes, A. Ferumoxytol-Enhanced MRI in Imaging Lymph Nodes in Patients with Locally Advanced Rectal Cancer; ClinicalTrials.gov Identifier: NCT03280277; OHSU Knight Cancer Institute: Portland, OR, USA, 2017. [Google Scholar]
- Choi, H. Clinical and Technical Feasibility of a Ultrasuperparamagnetic Nanoparticle Iron Oxide (USPIO)-Enhanced Magnetic Resonance Lymph; ClinicalTrials.gov Identifier: NCT01815333; University of Texas MD Anderson Cancer Center: Houston, TX, USA, 2013. [Google Scholar]
- Choyke, P.L. Ferumoxytol Enhanced MRI for the Detection of Lymph Node Involvement in Prostate Cancer; ClinicalTrials.gov Identifier: NCT01296139; National Cancer Institute (NCI): Rockville, MD, USA, 2011.
- Turkbey, I.B. Study of Ferumoxytol Enhanced MRI for Detecting Lymph Node Metastases in Prostate, Bladder, and Kidney Cancers; ClinicalTrials.gov Identifier: NCT02141490; National Cancer Institute (NCI): Rockville, MD, USA, 2014.
- Harisinghani, M. Magnetic Resonance Imaging of Lymph Nodes Using Ferumoxytol in Patients with Primary Prostate or Breast Cancer; ClinicalTrials.gov Identifier: NCT00087347; Massachusetts General Hospital Cancer Center: Boston, MA, USA, 2004. [Google Scholar]
- Neuwelt, E. DSC-MRI with Ferumoxytol and DCE-MRI with Gadolinium in Imaging Vascular Properties in Younger Patients with Brain Tumors; ClinicalTrials.gov Identifier: NCT00978562; OHSU Knight Cancer Institute: Portland, OR, USA, 2009. [Google Scholar]
- Iv, M. Using Ferumoxytol-Enhanced MRI to Measure Inflammation in Patients with Brain Tumors or Other Conditions of the CNS; ClinicalTrials.gov Identifier: NCT02452216; Stanford University Hospitals and Clinics: Stanford, CA, USA, 2015. [Google Scholar]
- Iv, M. In Vivo Characterization of Macrophages in Pediatric Patients with Malignant Brain Tumors Using Ferumoxytol-Enhanced MRI; ClinicalTrials.gov Identifier: NCT03179449; Stanford University Hospitals and Clinics: Stanford, CA, USA, 2017. [Google Scholar]
- Neuwelt, E. Ferumoxytol in Improving MR Imaging in Patients with High-Grade Brain Tumors or Cerebral Metastases; ClinicalTrials.gov Identifier: NCT00103038; OHSU Knight Cancer Institute: Portland, OR, USA, 2005. [Google Scholar]
- Seyfer, P.; Pagenstecher, A.; Mandic, R.; Klose, K.J.; Heverhagen, J.T. Cancer and inflammation: Differentiation by USPIO-enhanced MR imaging. J. Magn. Reson. Imaging 2014, 39, 665–672. [Google Scholar] [CrossRef]
- Daldrup-Link, H.E.; Marina, N. Differentiation of Bone Sarcomas and Osteomyelitis with Ferumoxytol-Enhanced MRI; ClinicalTrials.gov Identifier: NCT01336803; Stanford University School of Medicine: Stanford, CA, USA, 2011. [Google Scholar]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Neuwelt, E. Ferumoxytol MRI in Assessing Response to Pembrolizumab in Patients with Brain Tumors from Melanoma and Glioblastoma; ClinicalTrials.gov Identifier: NCT03347617; OHSU Knight Cancer Institute: Portland, OR, USA, 2017. [Google Scholar]
- Neuwelt, E. MRI Study with Ferumoxytol in Assessing Early Response in Patients with Glioblastoma Multiforme Receiving Temozolomide and Radiation Therapy; ClinicalTrials.gov Identifier: NCT00660543; OHSU Knight Cancer Institute: Portland, OR, USA, 2008. [Google Scholar]
- Neuwelt, E. Assessing Dynamic Magnetic Resonance (MR) Imaging in Patients with Recurrent High Grade Glioma Receiving Chemotherapy; ClinicalTrials.gov Identifier: NCT00769093; OHSU Knight Cancer Institute: Portland, OR, USA, 2008. [Google Scholar]
- Newby, D.E. Ferumoxytol for Magnetic Resonance Imaging of Myocardial; ClinicalTrials.gov Identifier: NCT01323296; Royal Infirmary of Edinburgh: Edinburgh, UK, 2011. [Google Scholar]
- Sigalov, V. MRA with Feraheme in HHT; ClinicalTrials.gov Identifier: NCT02977637; University of California: California, CA, USA, 2016. [Google Scholar]
- Jackson, R.A. Imaging Inflammation in Autoimmune Diabetes; ClinicalTrials.gov Identifier: NCT00585936; Joslin Diabetes Cente: Boston, MA, USA, 2008. [Google Scholar]
- Gaglia, J. Imaging of Type 1 Diabetes Progression; ClinicalTrials.gov Identifier: NCT01521520; Massachusetts General Hospital: Boston, MA, USA, 2012. [Google Scholar]
- Daldrup-Link, H.E. Imaging Kidney Transplant Rejection Using Ferumoxytol-Enhanced Magnetic Resonance; ClinicalTrials.gov Identifier: NCT02006108; Lucile Packard Children’s Hospital: Palo Alto, CA, USA, 2013. [Google Scholar]
- Daldrup-Link, H.E. Imaging of Osteonecrosis with Ferumoxytol-Enhanced MRI; ClinicalTrials.gov Identifier: NCT02893293; Lucile Packard Children’s Hospital: Palo Alto, CA, USA, 2016. [Google Scholar]
- Neuwelt, E. Gadolinium and Ferumoxytol MRI in Diagnosing with Abnormalities in the Central Nervous System; ClinicalTrials.gov Identifier: NCT03270059; OHSU Knight Cancer Institute: Portland, OR, USA, 2017. [Google Scholar]
- Reich, D.S. In Vivo Characterization of Inflammation with Ferumoxytol, an Ultrasmall Superparamagnetic Iron Oxide Nanoparticle, on 7 Tesla Magnetic Resonance Imaging; ClinicalTrials.gov Identifier: NCT02511028; National Institutes of Health Clinical Center: Bethesda, MD, USA, 2015. [Google Scholar]
- Jobst, B.C. Imaging Neuroinflammation in Epilepsy with Ferumoxytol MRI (IRONMAN); ClinicalTrials.gov Identifier: NCT02084303; Dartmouth-Hitchcock Medical Center: Lebanon, NH, USA, 2014. [Google Scholar]
- Nakamoto, B.K. Ferumoxytol-Enhanced Brain MRI in HIV-Associated Neurocognitive Disorders; ClinicalTrials.gov Identifier: NCT01665846; Hawaii Center for AIDS: Honolulu, HI, USA, 2012. [Google Scholar]
- Nakamoto, B.K. Ferumoxytol-Enhanced Imaging and Mapping in neuroAIDS; ClinicalTrials.gov Identifier: NCT02678767; Hawaii Center for AIDS: Honolulu, HI, USA, 2016. [Google Scholar]
- Stoumpos, S. Ferumoxytol Enhanced Magnetic Resonance Angiography in Chronic Kidney Disease (FeMRA in CKD); ClinicalTrials.gov Identifier: NCT02997046; NHS Greater Glasgow and Clyde: Glasgow, UK, 2016. [Google Scholar]
- Strauss, W. Magnetic Resonance Angiography for Peripheral Arterial Disease (PAD); ClinicalTrials.gov Identifier: NCT00707876; AMAG Pharmaceuticals, Inc.: Waltham, MA, USA, 2008. [Google Scholar]
- Sridhar, S. Quantitative Non-Invasive Brain Imaging Using QUTE-CE MRI; ClinicalTrials.gov Identifier: NCT0326684; Massachusetts General Hospital: Boston, MA, USA, 2017. [Google Scholar]
- Fawzy, M. Magnetic Nanoparticle Sperm Separation for Teratozoospermia Male and Women Older than 35 Years; ClinicalTrials.gov Identifier: NCT03666364; Ibn Sina Hospital (EGY): Giza, Egypt, 2018. [Google Scholar]
- Guimaraes, A. Ferumoxytol-Enhanced MRI in Imaging Lymph Nodes in Patients with Stage IIB-IIC Esophageal Cancer; ClinicalTrials.gov Identifier: NCT02857218; OHSU Knight Cancer Institute: Portland, OR, USA, 2016. [Google Scholar]
- Wu, Y.-W. Magnetic Nanoparticles System in Acute Coronary Syndrome; ClinicalTrials.gov Identifier: NCT02226523; Far Eastern Memorial Hospital (TWN): New Taipei City, Taiwan, 2014. [Google Scholar]
- Ambady, P. Pembrolizumab and Magnetic Resonance Imaging with Ferumoxytol in Treating Patients with Non-Small Cell Lung Cancer and Brain; ClinicalTrials.gov Identifier: NCT03325166; OHSU Knight Cancer Institute: Portland, OR, USA, 2017. [Google Scholar]
- Neuwelt, E. Ferumoxytol in Magnetic Resonance Imaging of Pediatric Patients with Brain Tumors; ClinicalTrials.gov Identifier: NCT03234309; OHSU Knight Cancer Institute: Portland, OR, USA, 2017. [Google Scholar]
- Neuwelt, E. MR, Histologic and EM Imaging of Intravenous Ferumoxytol in Central Nervous System (CNS) Inflammation; ClinicalTrials.gov Identifier: NCT00659776; OHSU Knight Cancer Institute: Portland, OR, USA, 2008. [Google Scholar]
- Stirrat, C. Detection of Cellular Inflammation with Ferumoxytol in the HEART (DECIFER); ClinicalTrials.gov Identifier: NCT02319278; Royal Infirmary of Edinburgh: Edinburgh, UK, 2014. [Google Scholar]
- Finn, P. Feraheme as an MRI Contrast Agent for Pediatric Congenital Heart Disease; ClinicalTrials.gov Identifier: NCT02752191; UCLA Medical Center: Los Angele, CA, USA, 2016. [Google Scholar]
- Siedlecki, A.M. Ferumoxytol for Magnetic Resonance Imaging in Patients with Severe Kidney Disease; ClinicalTrials.gov Identifier: NCT02954510; Brigham and Women’s Hospital: Boston, MA, USA, 2016. [Google Scholar]
- Kharlamov, A. Plasmonic Photothermal and Stem Cell Therapy of Atherosclerosis Versus Stenting (NANOM PCI); ClinicalTrials.gov Identifier: NCT01436123; De Haar Research Foundation (NLD): Utrecht, The Netherlands, 2011. [Google Scholar]
- Newby, D.E. Metabolic Imaging in Carotid Atherosclerosis (MICA); ClinicalTrials.gov Identifier: NCT01674257; University of Edinburgh: Edinburgh, UK, 2012. [Google Scholar]
- Kastrup, J. In Vivo Tracking of USPIO Labeled MSC in the Heart (USPIO-MSC); ClinicalTrials.gov Identifier: NCT03651791; Rigshospitalet (DNK): København, Danmark, 2018. [Google Scholar]
- Agarwal, R. Iron deficiency anemia in chronic kidney disease: Uncertainties and cautions. Hemodial. Int. 2017, 21 (Suppl. 1), S78–S82. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Strauss, W.E.; McLaughlin, J.; Li, Z.; Dellanna, F.; Hertel, J. A randomized comparison of ferumoxytol and iron sucrose for treating iron deficiency anemia in patients with CKD. Clin. J. Am. Soc. Nephrol. CJASN 2014, 9, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M. A Study to Evaluate the Safety (Compared to Iron Sucrose), Efficacy and Pharmacokinetics of Ferumoxytol for the Treatment of Iron Deficiency Anemia (IDA) in Pediatric Subjects with Chronic Kidney Disease (CKD); ClinicalTrials.gov Identifier: NCT03619850; AMAG Pharmaceuticals, Inc.: Waltham, MA, USA, 2018. [Google Scholar]
- Hill, M. Intravenous Infusions of Ferumoxytol Compared to Oral Ferrous Sulfate for the Treatment of Anemia in Pregnancy; ClinicalTrials.gov Identifier: NCT03657433; University of Arizona: Tucson, AZ, USA, 2018. [Google Scholar]
- Lu, M.; Cohen, M.H.; Rieves, D.; Pazdur, R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am. J. Hematol. 2010, 85, 315–319. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/rienso (accessed on 30 November 2018).
- Adkinson, N.F.; Strauss, W.E.; Macdougall, I.C.; Bernard, K.E.; Auerbach, M.; Kaper, R.F.; Chertow, G.M.; Krop, J.S. Comparative safety of intravenous ferumoxytol versus ferric carboxymaltose in iron deficiency anemia: A randomized trial. Am. J. Hematol. 2018, 93, 683–690. [Google Scholar] [CrossRef] [PubMed]
- A Phase IV Trial of Repeated Doses of Ferumoxytol Compared to Iron Sucrose for the Treatment of Iron Deficiency Anemia in Patients with Chronic Kidney Disease on Hemodialysis (FACT); ClinicalTrials.gov Identifier: NCT01227616; AMAG Pharmaceuticals, Inc.: Waltham, MA, USA, 2010.
- Gabinsky, J.; Kovtun, O.; Kharlamov, A. Plasmonic Nanophotothermal Therapy of Atherosclerosis (NANOM-FIM); ClinicalTrials.gov Identifier: NCT01270139; De Haar Research Foundation (NLD): Utrecht, The Netherlands, 2011. [Google Scholar]
- Pell, J.C.; Stenson, R. Enhanced Epidermal Antigen Specific Immunotherapy trial-1 (EE-ASI-1); ClinicalTrials.gov Identifier: NCT02837094; Cardiff University: Cardiff, UK, 2016. [Google Scholar]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; Waldöfner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.A.; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperth. 2005, 21, 637–647. [Google Scholar] [CrossRef]
- Wust, P.; Gneveckow, U.; Johannsen, M.; Bohmer, D.; Henkel, T.; Kahmann, F.; Sehouli, J.; Felix, R.; Ricke, J.; Jordan, A. Magnetic nanoparticles for interstitial thermotherapy-feasibility, tolerance and achieved temperatures. Int. J. Hyperth. 2006, 22, 673–685. [Google Scholar] [CrossRef]
- Johannsen, M.; Thiesen, B.; Wust, P.; Jordan, A. Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperth. 2010, 26, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H. Magnetic Nanoparticle Thermoablation-Retention and Maintenance in the Prostate: A Phase 0 Study in Men (MAGNABLATE I); ClinicalTrials.gov Identifier: NCT02033447; University College London Hospital: London, UK, 2014. [Google Scholar]
- Swenson, C.E.; Haemmerich, D.; Maul, D.H.; Knox, B.; Ehrhart, N.; Reed, R.A. Increased duration of heating boosts local drug deposition during radiofrequency ablation in combination with thermally sensitive liposomes (ThermoDox) in a porcine model. PLoS ONE 2015, 10, e0139752. [Google Scholar] [CrossRef] [PubMed]
- Middleton, M. Targeted Chemotherapy Using Focused Ultrasound for Liver Tumours (TARDOX); ClinicalTrials.gov Identifier: NCT02181075; Oxford University Hospitals NHS Trust: Oxford, UK, 2014. [Google Scholar]
- Lyon, P.C.; Griffiths, L.F.; Lee, J.; Chung, D.; Carlisle, R.; Wu, F.; Middleton, M.R.; Gleeson, F.V.; Coussios, C.C. Clinical trial protocol for TARDOX: A phase I study to investigate the feasibility of targeted release of lyso-thermosensitive liposomal doxorubicin (ThermoDox®) using focused ultrasound in patients with liver tumours. J. Ther. Ultrasound 2017, 5, 28. [Google Scholar] [CrossRef]
- Lyon, P.C.; Gray, M.D.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.F.; Scott, S.; Anderson, M.; Goldin, R.; et al. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): A single-centre, open-label, phase 1 trial. Lancet Oncol. 2018. [Google Scholar] [CrossRef]
- Stern, E. A Phase I Study of Lyso-Thermosensitive Liposomal Doxorubicin and MR-HIFU for Pediatric Refractory Solid Tumors; ClinicalTrials.gov Identifier: NCT02536183; Children’s National Medical Center: Washington, DC, USA, 2015. [Google Scholar]
- Borys, N. Phase II Study of Thermodox as Adjuvant Therapy with Thermal Ablation (RFA) in Treatment of Metastatic Colorectal Cancer (mCRC); ClinicalTrials.gov Identifier: NCT01464593; Celsion: Lawrenceville, NJ, USA, 2011. [Google Scholar]
- Lencioni, R.; Ping Poon, R.T.; Hua, C.M. Study of Thermodox with Standardized Radiofrequency Ablation (RFA) for Treatment of Hepatocellular Carcinoma (HCC) (OPTIMA); ClinicalTrials.gov Identifier: NCT02112656; Celsion: Lawrenceville, NJ, USA, 2014. [Google Scholar]
- Borys, N. Phase ½ Study of Thermodox with Approved Hyperthermia in Treatment of Breast Cancer Recurrence at the Chest Wall (DIGNITY); ClinicalTrials.gov Identifier: NCT00826085; Celsion: Lawrenceville, NJ, USA, 2009. [Google Scholar]
- de Maar, J.S.; Suelmann, B.B.M. Image-Guided Targeted Doxorubicin Delivery with Hyperthermia To Optimize Loco-Regional Control in Breast Cancer (i-GO); ClinicalTrials.gov Identifier: NCT03749850; UMC Utrecht (NLD): Utrecht, The Netherlands, 2018. [Google Scholar]
- A Trial of Ferumoxytol for the Treatment of Iron Deficiency Anemia in Pediatric Participants with Nondialysis-Dependent Chronic Kidney Disease; ClinicalTrials.gov Identifier: NCT01155388; AMAG Pharmaceuticals, Inc.: Waltham, MA, USA, 2010.
- Mahajan, M.; Utreja, P.; Jain, S.K. Paclitaxel loaded nanoliposomes in thermosensitive hydrogel: A dual approach for sustained and localized delivery. Anti-Cancer Agents Med. Chem. 2016, 16, 365–376. [Google Scholar] [CrossRef]
- Agrahari, V.; Patel, S.P.; Dhall, N.; Aulgur, Z.; Thukral, S.; Yang, X.; Conley, R.; Mitra, A.K. Nanoparticles in thermosensitive gel based composite nanosystem for ocular diseases. Drug Deliv. Transl. Res. 2018, 8, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moreno, P.; Ortega-Vinuesa, J.L.; Peula-Garcia, J.M.; Marchal, J.A.; Boulaiz, H. Smart drug-delivery systems for cancer nanotherapy. Curr. Drug Targets 2018, 19, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Venditto, V.J.; Szoka, F.C., Jr. Cancer nanomedicines: So many papers and so few drugs! Adv. Drug Deliv. Rev. 2013, 65, 80–88. [Google Scholar] [CrossRef]



| Type of TSLs | Drug | Improvement | Use | Ref. |
|---|---|---|---|---|
| Liposome formulations of HTLC | HTLC | Treatment of cervical carcinoma | Treatment | [56] |
| Liposomal formulations with drug | GEM DOX | Increased drug plasma half-life | Treatment | [55] [65] |
| Multi-modal thermo-sensitive polymer-modified liposomes (MTPLs) | MnSO4, rhodamine, DOX | Monitor drug delivery in cancer therapy | Diagnosis | [66] |
| Liposomal ICG | ICG | Deliver ICG as the NIR in the treatment of triple negative breast cancer | Treatment | [67] |
| TSLs loaded with drug and fullerene decorated with IONs | ICG, DOX | Multifunctional TSLs showing radiofrequency release and MRI for tumor therapy | Diagnosis and treatment | [60] |
| TSLs loaded with drugs and mesoporous silica nanoparticles | ICG, DOX | Multifunctional nanoplatform for integrate diagnosis and treatment (photodynamic therapy and PTT) for cancer | Diagnosis and treatment | [68] |
| Pathology | Interventions | ClinicalTrials ID | Phase | Date (First–Last Posted) | Ref. |
|---|---|---|---|---|---|
| Blood Biomarkers Vasodilation Blood Clotting Lung Function Healthy Volunteers | - Diesel exhaust particulate | NCT03659864 | Not applicable | September 2018–still active | [69] |
| - Carbon nanoparticles | |||||
| - Small graphene oxide | |||||
| - Ultrasmall graphene oxide | |||||
| Metastatic and Primary Brain Neoplasm | - Ferumoxytol | NCT00659126 | Phase 2 | 2018–still active | [74] |
| - 3 Tesla MRI | |||||
| - Dynamic Contrast-Enhanced MRI | |||||
| Head and Neck Cancer | - Ferumoxytol | NCT01895829 | Early Phase | 2013–still active | [75] |
| - MRI | |||||
| Solid Tumors | Ferumoxytol followed by MM-398 | NCT01770353 | Phase 1 | 2013–still active | [76] |
| ER/PR Positive Breast Cancer | |||||
| Active Brain Metastasis | |||||
| Rectal Cancer (Stage III) Esophageal Cancer (Stage II–III) | - Ferumoxytol - MRI - PET/CT | NCT03280277 | Phase 2 | 2017–still active | [77] |
| Cancer of Lymph Node | - Feraheme | NCT01815333 | Not applicable | 2013–still active | [78] |
| - MRI | |||||
| Prostate Cancer | Drug: Ferumoxytol | NCT01296139 | Phase 1 | 2011–2018 | [79] |
| Prostate Cancer Bladder Cancer Kidney Cancer | - Ferumoxytol - MRI | NCT02141490 | Phase 2 | 2014–still active | [80] |
| Breast Cancer | - Ferumoxytol | NCT00087347 | Not applicable | 2013–still active | [81] |
| Prostate Cancer | - MRI | ||||
| Soft Tissue Sarcoma | - Ferumoxytol | NCT00978562 | Not applicable | 2017–still active | [82] |
| Childhood Brain Neoplasm | - MRI | ||||
| Brain Injury | - Ferumoxytol | NCT02452216 | Early Phase 1 | 2015–2017 | [83] |
| CNS: Degenerative and Infectious Disorder | - MRI | ||||
| Childhood Brain Neoplasm | - Ferumoxytol | NCT03179449 | Early Phase 1 | 2017–still active | [84] |
| - MRI | |||||
| CNS Brain Neoplasm | - Ferumoxytol - 3 Tesla MRI - Dynamic MRI | NCT02857218 NCT00103038 | Not applicable | 2005–still active | [107] [85] |
| Bone Cancer Chondrosarcoma Ewing’s Sarcoma | - Feraheme - MRI | NCT01336803 | Not applicable | 2011–still active | [87] |
| Glioblastoma | - Pembrolizumab | NCT03347617 | Phase 2 | 2017–still active | [89] |
| Malignant Primary and metastatic Brain Neoplasm | - Ferumoxytol | ||||
| Melanoma | - MRI | ||||
| Adult Brain Glioblastoma | - Gadolinium | NCT00660543 | Not applicable | 2016–2017 | [90] |
| - Ferumoxytol | |||||
| - MRI | |||||
| Brain Neoplasms | Ferumoxytol | NCT00769093 | Phase 1 | 2008–2017 | [91] |
| Myocardial Infarction | - Ferumoxytol | NCT01323296 | Not applicable | 2011–2014 | [92] |
| - MRI | |||||
| Hereditary Hemorrhagic Telangiectasia | Feraheme MRI/MRA | NCT02977637 | Phase 1 | 2016–still active | [93] |
| Diabetes Mellitus, Type 1 | - Ferumoxytol | NCT00585936 | Not applicable | 2008–2011 | [94] |
| - MRI | |||||
| Type 1 Diabetes | - Ferumoxytol | NCT01521520 | Not applicable | 2012–still active | [95] |
| - MRI | |||||
| Renal Transplant Rejection | - Feraheme | NCT02006108 | Not applicable | 2017–2018 | [96] |
| - MRI-GE Healthcare 3 Tesla magnet | |||||
| Osteonecrosis | - Ferumoxytol | NCT02893293 | Phase 4 | 2015–still active | [97] |
| - MRI | |||||
| CNS Neoplasm | - Ferumoxytol | NCT03270059 | Phase 2 | 2017–still active | [98] |
| - Gadolinium | |||||
| - MRI | |||||
| MS | - Ferumoxytol | NCT02511028 | Early Phase 1 | 2015–still active | [99] |
| - MRI | |||||
| Epilepsy | - Ferumoxytol injection after focal epileptic seizure | NCT02084303 | Not applicable | 2014–2018 | [100] |
| - MRI | |||||
| HIV Dementia | - Ferumoxytol | NCT01665846 | Phase 1 | 2012–2018 | [101] |
| AIDS Dementia Complex | - Ferumoxytol | NCT02678767 | 2016-2017 | Phase 2 | [102] |
| - MRI | |||||
| Peripheral Arterial Disease | - Ferumoxytol | NCT00707876 | Phase 2 | 2008–still active | [104] |
| - MRI | |||||
| Cerebrovascular Disorders | Quantitative MRI | NCT03266848 | Not applicable | 2017–still active | [105] |
| Infertility | MNP Sperm Separation for ICSI Cycles | NCT03666364 | Not applicable | September 2018–still active | [106] |
| Acute Coronary Syndrome | Immunomagnetic reduction by MNPs | NCT02226523 | Not applicable | 2014–still active | [108] |
| Lung Carcinoma | - Pembrolizumab | NCT03325166 | Phase 2 | 2017–still active | [109] |
| - Ferumoxytol | |||||
| - MRI | |||||
| Childhood Brain Neoplasm | - Ferumoxytol | NCT03234309 | Phase 2 | 2017–still active | [110] |
| - MRI | |||||
| Nervous System Diseases | - Ferumoxytol | NCT00659776 | Phase 2 | 2008–2012 | [111] |
| - MRI | |||||
| Cardiac Transplant Cardiac Sarcoid Myocarditis | - Ferumoxytol | NCT02319278 | Phase 2 | 2014–2017 | [112] |
| - MRI | Phase 3 | ||||
| Pediatric Congenital Heart Disease | - Ferumoxytol | NCT02752191 | Phase 4 | 2016–still active | [113] |
| - Gadofosveset | |||||
| Coronary Artery Disease | - Ferumoxytol | NCT02954510 | Phase 3 | 2016–still active | [114] |
| - MRI | |||||
| - Coronary Artery Disease - Atherosclerosis | - Stenting and micro -infusion of GNPs | NCT01436123 | Phase 1 | 2011–2015 | [115] |
| - Implantation of everolimus-eluting stent | |||||
| Atherosclerosis | - Ferumoxytol - MRI scan | NCT01674257 | Not applicable | 2012–2013 | [116] |
| Stroke | - Radiation: 18f-Fluoride PET/CT - Radiation: 18F-Flurodeoxyglucose PET/CT | ||||
| Ischemic Heart Disease | Iron oxide-labeled mesenchymal stromal | NCT03651791 | Phase 1 | August 2018–still active | [117] |
| MRI |
| Pathology | Interventions | ClinicalTrials ID | Phase | Date (First–Last Posted) | Ref. |
|---|---|---|---|---|---|
| CKD | - Ferumoxytol | NCT02997046 | Phase 4 | 2016‒still active | [104] |
| - MRA | |||||
| Ischemic Heart Disease | - Iron oxide-labeled mesenchymal stromal cells | NCT03651791 | Phase 1 | August 2018‒still active | [117] |
| - MRI | |||||
| IDA | - Ferumoxytol | NCT03619850 | Phase 3 | August 2018‒still active | [120] |
| Pediatric CKD | - Oral Iron | ||||
| IDA | Intravenous and Oral Iron | NCT03657433 | Phase 3 | September 2018 | [121] |
| Pregnancy | |||||
| IDA | - Ferumoxytol | NCT01227616 | Phase 4 | 2010‒2017 | [125] |
| CKD | - Iron Sucrose | ||||
| - Stable Angina - Heart Failure - Atherosclerosis - Multivessel Coronary Artery Disease | - GNPs - Iron-bearing nanoparticles - Stenting | NCT01270139 | Not applicable | 2012‒2017 | [126] |
| Type 1 Diabetes | - C19-A3 GNP (peptide fragment related to insulin attached to GNPs) | NCT02837094 | Phase 1 | 2016‒still active | [127] |
| Prostate Cancer | MNPs Injection | NCT02033447 | Early Phase 1 | 2013‒2017 | [131] |
| Liver Tumor | - ThermoDox® | NCT02181075 | Phase 1 | 2014‒2017 | [133] |
| - Magnetic resonance high intensity focused ultrasound | |||||
| Pediatric Cancer Solid Tumors | - ThermoDox® | NCT02536183 | Phase 1 | 2015‒still active | [136] |
| - MRI | |||||
| - High-intensity focused ultrasound | |||||
| Colon Cancer | - ThermoDox® | NCT01464593 | Phase 2 | 2011‒2016 | [137] |
| Liver Metastasis | - Radiofrequency ablation | ||||
| Hepatocellular Carcinoma | - ThermoDox® | NCT02112656 | Phase 3 | 2014‒still active | [138] |
| - Radiofrequency ablation | |||||
| Breast Cancer | - ThermoDox® | NCT00826085 | Phase 2 | 2009‒2017 | [139] |
| - Microwave hyperthermia | |||||
| Breast Cancer | - ThermoDox® | NCT03749850 | Phase 1 | November 2018‒still active | [140] |
| - MR-HIFU induced hyperthermia | |||||
| - Cyclophosphamide | |||||
| - IDA | - Ferumoxytol | NCT01155388 | Phase 3 | 2017‒still active | [141] |
| - Non-dialysis-dependent CKD | - Oral Iron |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardecchia, S.; Sánchez-Moreno, P.; de Vicente, J.; Marchal, J.A.; Boulaiz, H. Clinical Trials of Thermosensitive Nanomaterials: An Overview. Nanomaterials 2019, 9, 191. https://doi.org/10.3390/nano9020191
Nardecchia S, Sánchez-Moreno P, de Vicente J, Marchal JA, Boulaiz H. Clinical Trials of Thermosensitive Nanomaterials: An Overview. Nanomaterials. 2019; 9(2):191. https://doi.org/10.3390/nano9020191
Chicago/Turabian StyleNardecchia, Stefania, Paola Sánchez-Moreno, Juan de Vicente, Juan A. Marchal, and Houria Boulaiz. 2019. "Clinical Trials of Thermosensitive Nanomaterials: An Overview" Nanomaterials 9, no. 2: 191. https://doi.org/10.3390/nano9020191