Calcium Pyruvate Exerts Beneficial Effects in an Experimental Model of Irritable Bowel Disease Induced by DCA in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
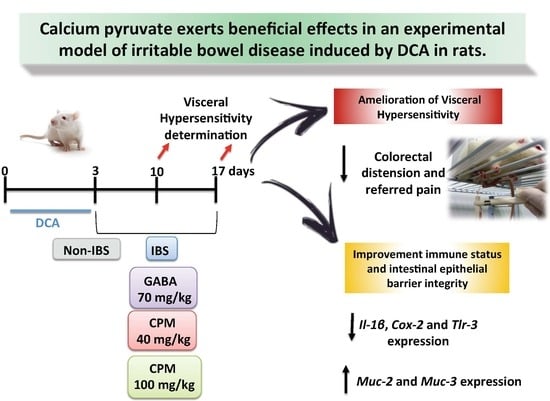
2.2. Rat Model of Chronic Post-Inflammatory Visceral Pain Induced by DCA
2.3. Measurement of Response to Colorectal Distension
2.4. Determination of Referred Pain
2.5. In Vivo Intestinal Permeability
2.6. Statistics
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fink, M.P. Ethyl pyruvate. Curr. Opin. Anaesthesiol. 2008, 21, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Pischel, I.; Weiss, S.; Ortenburger, G.; Konig, H. Method of Producing Calcium Pyruvates. US Patent 5962734, 5 October 1999. [Google Scholar]
- De Oliveira Freitas, D.M.; Stampini Duarte Martino, H.; Machado Rocha Ribeiro, S.; Goncalves Alfenas, R.C. Calcium ingestion and obesity control. Nutr. Hosp. 2012, 27, 1758–1771. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.J.; Wood, R.; Kim, K. Calcium supplementation. J. Am. Acad. Nurse Pract. 1997, 9, 187–192. [Google Scholar] [CrossRef]
- Algieri, F.; Rodriguez-Nogales, A.; Garrido-Mesa, J.; Camuesco, D.; Vezza, T.; Garrido-Mesa, N.; Utrilla, P.; Rodriguez-Cabezas, M.E.; Pischel, I.; Galvez, J. Intestinal anti-inflammatory activity of calcium pyruvate in the TNBS model of rat colitis: Comparison with ethyl pyruvate. Biochem. Pharmacol. 2016, 103, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bohn, L.; Storsrud, S.; Simren, M. Nutrient intake in patients with irritable bowel syndrome compared with the general population. Neurogastroenterol. Motil. 2013, 25. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; Hausken, T.; Gundersen, D.; El-Salhy, M. Effects of dietary guidance on the symptoms, quality of life and habitual dietary intake of patients with irritable bowel syndrome. Mol. Med. Rep. 2013, 8, 845–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, M.J.; Sabate, J.M.; Bouchoucha, M.; Buscail, C.; Hercberg, S.; Julia, C. Food consumption and dietary intakes in 36,448 adults and their association with irritable bowel syndrome: Nutrinet-Sante study. Therap. Adv. Gastroenterol. 2018, 11, 1756283X17746625. [Google Scholar] [CrossRef]
- Stobaugh, D.J.; Deepak, P.; Ehrenpreis, E.D. Increased risk of osteoporosis-related fractures in patients with irritable bowel syndrome. Osteoporos. Int. A J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2013, 24, 1169–1175. [Google Scholar] [CrossRef]
- Vesa, T.H.; Seppo, L.M.; Marteau, P.R.; Sahi, T.; Korpela, R. Role of irritable bowel syndrome in subjective lactose intolerance. Am. J. Clin. Nutr. 1998, 67, 710–715. [Google Scholar] [CrossRef]
- Ostgaard, H.; Hausken, T.; Gundersen, D.; El-Salhy, M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol. Med. Rep. 2012, 5, 1382–1390. [Google Scholar] [CrossRef]
- Barbara, G.; Cremon, C.; Carini, G.; Bellacosa, L.; Zecchi, L.; De Giorgio, R.; Corinaldesi, R.; Stanghellini, V. The immune system in irritable bowel syndrome. J. Neurogastroenterol. Motil. 2011, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Ostgaard, H.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. The role of diet in the pathogenesis and management of irritable bowel syndrome (Review). Int. J. Mol. Med. 2012, 29, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Cipriani, P.; Carubbi, F.; Liakouli, V.; Zazzeroni, F.; Di Benedetto, P.; Berardicurti, O.; Alesse, E.; Giacomelli, R. The role of IL-1beta in the bone loss during rheumatic diseases. Mediat. Inflamm. 2015, 782382. [Google Scholar] [CrossRef]
- Ford, A.C.; Talley, N.J. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: A systematic review. J. Gastroenterol. 2011, 46, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Adam, B.; Tsopelas, C.; Liebregts, T.; Bartholomeusz, F.D.; Holtmann, G. Host immune response determines visceral hyperalgesia in a rat model of post-inflammatory irritable bowel syndrome. J. Gastroenterol. 2013, 48, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.L. Inflammatory bowel diseases, celiac disease, and bone. Arch. Biochem. Biophys. 2010, 503, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Gambaccini, D.; Stasi, C.; Urbano, M.T.; Marchi, S.; Usai-Satta, P. Irritable bowel syndrome: A disease still searching for pathogenesis, diagnosis and therapy. World J. Gastroenterol. 2014, 20, 8807–8820. [Google Scholar] [CrossRef]
- Rzymski, P.; Pischel, I.; Conrad, F.; Zwingers, T.; Rzymski, P.; Opala, T. The bioavailability of calcium in the form of pyruvate, carbonate, citrate–malate in healthy postmenopausal women. Eur. Food Res. Technol. 2016, 242, 45–50. [Google Scholar] [CrossRef]
- Traub, R.J.; Tang, B.; Ji, Y.; Pandya, S.; Yfantis, H.; Sun, Y. A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology 2008, 135, 2075–2083. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; Group, N.C.R.R.G.W. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [Green Version]
- McGrath, J.C.; Drummond, G.B.; McLachlan, E.M.; Kilkenny, C.; Wainwright, C.L. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [PubMed]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Gálvez, J.; Zarzuelo, A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef]
- Zhang, C.; Rui, Y.Y.; Zhou, Y.Y.; Ju, Z.; Zhang, H.H.; Hu, C.Y.; Xiao, Y.; Xu, G.Y. Adrenergic beta2-receptors mediates visceral hypersensitivity induced by heterotypic intermittent stress in rats. PLoS ONE 2014, 9, e94726. [Google Scholar] [CrossRef]
- Keszthelyi, D.; Troost, F.J.; Masclee, A.A. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G141–G154. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Algieri, F.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Utrilla, M.P.; Talhaoui, N.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Rodríguez-Cabezas, M.E.; Monteleone, G.; et al. Immunomodulatory properties of Olea europaea leaf extract in intestinal inflammation. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Kim, J.H.; Cho, S.W. Gabapentin reduces rectal mechanosensitivity and increases rectal compliance in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2005, 22, 981–988. [Google Scholar] [CrossRef]
- Chadwick, V.S.; Chen, W.; Shu, D.; Paulus, B.; Bethwaite, P.; Tie, A.; Wilson, I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002, 122, 1778–1783. [Google Scholar] [CrossRef]
- Gwee, K.A.; Collins, S.M.; Read, N.W.; Rajnakova, A.; Deng, Y.; Graham, J.C.; McKendrick, M.W.; Moochhala, S.M. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut 2003, 52, 523–526. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.M.; Fu, Y.; Wu, C.C.; Xu, G.Y.; Huang, L.Y.; Shi, X.Z. Colon distention induces persistent visceral hypersensitivity by mechanotranscription of pain mediators in colonic smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G434–G441. [Google Scholar] [CrossRef] [Green Version]
- Martin-Viñas, J.J.; Quigley, E.M.J. Immune response in irritable bowel syndrome: A systematic review of systemic and mucosal inflammatory mediators. Dig. Dis. 2016, 17, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Hobson, A.R.; Hughes, A.; Growcott, J.; Woolf, C.J.; Thompson, D.G.; Aziz, Q. The prostaglandin E2 receptor-1 (EP-1) mediates acid-induced visceral pain hypersensitivity in humans. Gastroenterology 2003, 124, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Matricon, J.; Meleine, M.; Gelot, A.; Piche, T.; Dapoigny, M.; Muller, E.; Ardid, D. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012, 36, 1009–1031. [Google Scholar] [CrossRef] [PubMed]
- Piche, T. Tight junctions and IBS—The link between epithelial permeability, low-grade inflammation, and symptom generation? Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2014, 26, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, B.; Verne, G.N. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009, 146, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Barouei, J.; Moussavi, M.; Hodgson, D.M. Perinatal maternal probiotic intervention impacts immune responses and ileal mucin gene expression in a rat model of irritable bowel syndrome. Benef. Microbes 2015, 6, 83–95. [Google Scholar] [CrossRef]
- Da Silva, S.; Robbe-Masselot, C.; Ait-Belgnaoui, A.; Mancuso, A.; Mercade-Loubiere, M.; Salvador-Cartier, C.; Gillet, M.; Ferrier, L.; Loubiere, P.; Dague, E.; et al. Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: Prevention by a probiotic treatment. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G420–G429. [Google Scholar] [CrossRef]
- McKernan, D.P.; Nolan, A.; Brint, E.K.; O’Mahony, S.M.; Hyland, N.P.; Cryan, J.F.; Dinan, T.G. Toll-like receptor mRNA expression is selectively increased in the colonic mucosa of two animal models relevant to irritable bowel syndrome. PLoS ONE 2009, 4, e8226. [Google Scholar] [CrossRef]
- McKernan, D.P.; Gaszner, G.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2011, 33, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Chalazonitis, A.; Huang, Y.Y.; Mann, J.J.; Margolis, K.G.; Yang, Q.M.; Kim, D.O.; Cote, F.; Mallet, J.; Gershon, M.D. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 8998–9009. [Google Scholar] [CrossRef]
- Yan, C.; Xin-Guang, L.; Hua-Hong, W.; Jun-Xia, L.; Yi-Xuan, L. Effect of the 5-HT4 receptor and serotonin transporter on visceral hypersensitivity in rats. Braz. J. Med Biol. Res. 2012, 45, 948–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Gene | Sequence 5′–3′ | Annealing Temperature (°C) |
|---|---|---|
| Gapdh | FW: CCATCACCATCTTCCAGGAG RV: CCTGCTTCACCACCTTCTTG | 60 |
| Il-1β | FW: GATCTTTGAAGAAGAGCCCG RV: AACTATGTCCCGACCATTGC | 59 |
| Muc-2 | FW: ACCACCATTACCACCACCTCAG RV: CGATCACCACCATTGCCACTG | 60 |
| Muc-3 | FW: CACAAAGGCAAGAGTCCAGA RV: ACTGTCCTTGGTGCTGCTGAATG | 60 |
| Cox-2 | FW: TGATGACTGCCCAACTCCCATG RV: AATGTTGAAGGTGTCCGGCAGC | 60 |
| Tlr-3 | FW: GATTGGCAAGTTATTCGTC RV: GCGGAGGCTGTTGTAGG | 60 |
| Htr-4 | FW: CAGTTGAAGTTGCCATCAGC RV: CGGCGAATTGGAGATGAACT | 60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Nogales, A.; Algieri, F.; Vezza, T.; Garrido-Mesa, J.; Molina-Tijeras, J.A.; Rodríguez-Cabezas, M.E.; Utrilla, M.P.; Pischel, I.; Gálvez, J. Calcium Pyruvate Exerts Beneficial Effects in an Experimental Model of Irritable Bowel Disease Induced by DCA in Rats. Nutrients 2019, 11, 140. https://doi.org/10.3390/nu11010140
Rodríguez-Nogales A, Algieri F, Vezza T, Garrido-Mesa J, Molina-Tijeras JA, Rodríguez-Cabezas ME, Utrilla MP, Pischel I, Gálvez J. Calcium Pyruvate Exerts Beneficial Effects in an Experimental Model of Irritable Bowel Disease Induced by DCA in Rats. Nutrients. 2019; 11(1):140. https://doi.org/10.3390/nu11010140
Chicago/Turabian StyleRodríguez-Nogales, Alba, Francesca Algieri, Teresa Vezza, José Garrido-Mesa, José Alberto Molina-Tijeras, María Elena Rodríguez-Cabezas, María Pilar Utrilla, Ivo Pischel, and Julio Gálvez. 2019. "Calcium Pyruvate Exerts Beneficial Effects in an Experimental Model of Irritable Bowel Disease Induced by DCA in Rats" Nutrients 11, no. 1: 140. https://doi.org/10.3390/nu11010140