Bone Protective Effect of Extra-Virgin Olive Oil Phenolic Compounds by Modulating Osteoblast Gene Expression
Abstract
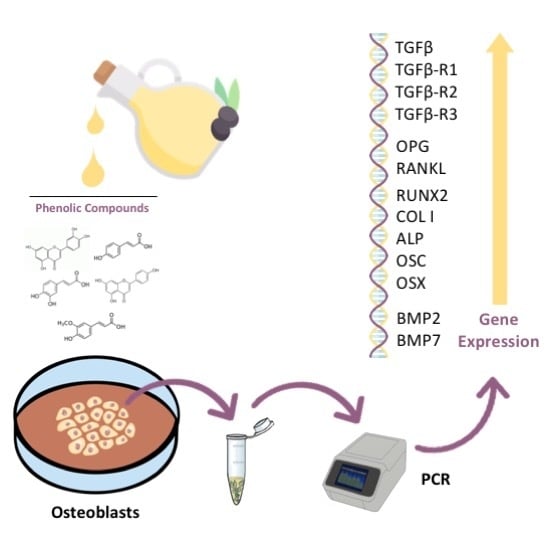
:1. Introduction
2. Material and Methods
2.1. Chemical Compounds
2.2. Cell Culture
2.3. Treatments
2.4. RNA Extraction and cDNA Synthesis (Reverse Transcription)
2.5. Real-Time Polymerase Chain Reaction (RT-PCR)
2.6. Statistical Analysis
3. Results
3.1. Effect of Phenolic Compounds on Gene Expression of TGFβ1 and its Receptors (TGFβ- R1, TGFβ-R2, and TGFβ-R3)
3.2. Effect of Phenolic Compounds on Gene Expression on BMP2 and BMP7
3.3. Effect of Phenolic Compounds on Gene Expression of OPG- RANKL Complex
3.4. Effect of Phenolic Compounds on the Gene Expression of RUNX-2, ALP, COL-I, OSX and OSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Poudyal, H.; Panchal, S.K. Functional foods as potential therapeutic options for metabolic syndrome. Obes. Rev. 2015, 16, 914–941. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, F.; Ruano, J.; Perez-Martinez, P.; Lopez-Segura, F.; Lopez-Miranda, J. The influence of olive oil on human health: Not a question of fat alone. Mol. Nutr. Food Res. 2007, 51, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, P.; Termentzi, A.; Michel, T.; Gikas, E.; Halabalaki, M.; Skaltsounis, A.-L. From olive drupes to olive oil. An HPLC-orbitrap-based qualitative and quantitative exploration of olive key metabolites. Planta Med. 2013, 79, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M. Concepts of Functional Food; International Life Sciences Institute (ILSI) Press: Washington, DC, USA, 2002; ISBN 157-8-81-1457. [Google Scholar]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic Compounds in Extra Virgin Olive Oil Stimulate Human Osteoblastic Cell Proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar] [CrossRef]
- Berrougui, H.; Ikhlef, S.; Khalil, A. Extra Virgin Olive Oil Polyphenols Promote Cholesterol Efflux and Improve HDL Functionality. Evid. Based Complement. Alternat. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casamenti, F.; Stefani, M. Olive polyphenols: New promising agents to combat aging-associated neurodegeneration. Expert Rev. Neurother. 2017, 17, 345–358. [Google Scholar] [CrossRef]
- Boss, A.; Bishop, K.; Marlow, G.; Barnett, M.; Ferguson, L. Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions. Nutrients 2016, 8, 513. [Google Scholar] [CrossRef]
- Bernabei, R.; Martone, A.M.; Ortolani, E.; Landi, F.; Marzetti, E. Screening, diagnosis and treatment of osteoporosis: A brief review. Clin. Cases Miner. Bone Metab. 2014, 11, 201–207. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of Osteoporosis at the Primary Health-Care Level; World Health Organization Collaborating Centre for Classification in Mental Health: Sydney, Australia; University of Sheffield: Sheffield, UK, 2009. [Google Scholar]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef]
- Seeman, E. Reduced bone formation and increased bone resorption: Rational targets for the treatment of osteoporosis. Osteoporos. Int. 2003, 14 (Suppl. 3), 2–8. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; Manzano-Moreno, F.J.; De Luna-Bertos, E.; Rivas, A.; Ramos-Torrecillas, J.; Ruiz, C.; García-Martínez, O. Effect of olive oil phenolic compounds on osteoblast differentiation. Eur. J. Clin. Investig. 2018, 48, e12904. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; Ramos-Torrecillas, J.; Manzano-Moreno, F.J.; Illescas-Montes, R.; Rivas, A.; Ruiz, C.; De Luna-Bertos, E.; García-Martínez, O. Effect of phenolic extracts from different extra-virgin olive oil varieties on osteoblast-like cells. PLoS ONE 2018, 13, e0196530. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.N.; Koohdani, F.; Eslaminejad, M.B.; Izadi, P.; Eshraghian, M.; Sayahpour, F.A.; Neek, L.S.; Shidfar, F. Extra virgin olive oil in maternal diet increases osteogenic genes expression, but high amounts have deleterious effects on bones in mice offspring at adolescence. Iran. J. Basic Med. Sci. 2016, 19, 1299–1307. [Google Scholar] [PubMed]
- Wu, X.; Li, Z.; Yang, Z.; Zheng, C.; Jing, J.; Chen, Y.; Ye, X.; Lian, X.; Qiu, W.; Yang, F.; et al. Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-κB ligand–induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+–nuclear factor of activated T-cells cytoplasmic 1 signaling pathways. J. Bone Miner. Res. 2012, 27, 1298–1308. [Google Scholar] [PubMed]
- Santiago-Mora, R.; Casado-Díaz, A.; De Castro, M.D.; Quesada-Gómez, J.M. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: The effect on differentiation in stem cells derived from bone marrow. Osteoporos. Int. 2011, 22, 675–684. [Google Scholar] [CrossRef]
- Ragni, E.; Viganò, M.; Rebulla, P.; Giordano, R.; Lazzari, L. What is beyond a qRT-PCR study on mesenchymal stem cell differentiation properties: How to choose the most reliable housekeeping genes. J. Cell. Mol. Med. 2013, 17, 168–180. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 1–9. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Medina-Huertas, R.; Ramos-Torrecillas, J.; García-Martínez, O.; Ruiz, C. The effect of low-level diode laser therapy on early differentiation of osteoblast via BMP-2/TGF-β1 and its receptors. J. Cranio Maxillofac. Surg. 2015, 43, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cao, Z.; Zhang, Q.; Li, M.; Han, L.; Li, Y. Aluminum trichloride inhibits osteoblast mineralization via TGF-β1/Smad signaling pathway. Chem. Biol. Interact. 2016, 244, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, H.; Okada, S.; Saito, A.; Hoshi, K.; Yamashita, H.; Takato, T.; Azuma, T. Inhibition of insulin-like growth factor-1 (IGF-1) expression by prolonged transforming growth factor-β1 (TGF-β1) administration suppresses osteoblast differentiation. J. Biol. Chem. 2012, 287, 22654–22661. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Saulacic, N.; Buser, D.; Iizuka, T.; Sculean, A. Osteoblast proliferation and differentiation on a barrier membrane in combination with BMP2 and TGFβ1. Clin. Oral Investig. 2013, 17, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Lieb, E.; Vogel, T.; Milz, S.; Dauner, M.; Schulz, M.B. Effects of transforming growth factor beta1 on bonelike tissue formation in three-dimensional cell culture. II: Osteoblastic differentiation. Tissue Eng. 2004, 10, 1414–1425. [Google Scholar] [PubMed]
- Chen, M.; Dong, Q.R.; Huang, Q.; Xu, W.; She, C. Effects of 0.5 Gy X-ray radiation on the profile of gene expression in MC3T3-E1 osteoblasts. J. Cranio Maxillofac. Surg. 2016, 96, 2659–2664. [Google Scholar]
- Wu, H.; Zha, Z.; Yao, P. Experimental study of icariin in inducing bone marrow mesenchymal stem cell differentiation. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi Chin. J. Integr. Tradit. West. Med. 2010, 30, 410–415. [Google Scholar]
- Canalis, E.; Economides, A.N.; Gazzerro, E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 2003, 24, 218–235. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Xu, B.; Wang, X.; Wu, C.; Zhu, L.; Chen, O.; Wang, X. Flavonoid compound icariin enhances BMP-2 induced differentiation and signalling by targeting to connective tissue growth factor (CTGF) in SAMP6 osteoblasts. PLoS ONE 2018, 13, e0200367. [Google Scholar] [CrossRef]
- Liang, W.; Lin, M.; Li, X.; Li, C.; Gao, B.; Gan, H.; Yang, Z.; Lin, X.; Liao, L.; Yang, M. Icariin promotes bone formation via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19 human osteoblastic cell line. Int. J. Mol. Med. 2012, 30, 889–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.-B.; Fong, Y.-C.; Tsai, H.-Y.; Chen, Y.-F.; Tsuzuki, M.; Tang, C.-H. Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur. J. Pharmacol. 2008, 588, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Gaoli, X.; Yi, L.; Lili, W.; Qiutao, S.; Guang, H.; Zhiyuan, G. Effect of naringin combined with bone morphogenetic protein-2 on the proliferation and differentiation of MC3T3-E1 cells. Hua Xi Kou Qiang Yi Xue Za Zhi Huaxi Kouqiang Yixue Zazhi West China J. Stomatol. 2017, 35, 275–280. [Google Scholar]
- Hu, B.; Yu, B.; Tang, D.; Li, S.; Wu, Y. Daidzein promotes osteoblast proliferation and differentiation in OCT1 cells through stimulating the activation of BMP-2/Smads pathway. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Hinoi, E.; Fujimori, S.; Wang, L.; Hojo, H.; Uno, K.; Yoneda, Y. Nrf2 negatively regulates osteoblast differentiation via interfering with Runx2-dependent transcriptional activation. J. Biol. Chem. 2006, 281, 18015–18024. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Xiao, G.; Jiang, D.; Gopalakrishnan, R.; Yang, S.; Reith, E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect. Tissue Res. 2003, 44 (Suppl. 1), 109–116. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Lee, C.-H.; Huang, Y.-L.; Liao, J.-F.; Chiou, W.-F. Ugonin K promotes osteoblastic differentiation and mineralization by activation of p38 MAPK- and ERK-mediated expression of Runx2 and osterix. Eur. J. Pharmacol. 2011, 668, 383–389. [Google Scholar] [CrossRef]
- Xu, D.; Xu, L.; Zhou, C.; Lee, W.Y.W.; Wu, T.; Cui, L.; Li, G. Salvianolic acid B promotes osteogenesis of human mesenchymal stem cells through activating ERK signaling pathway. Int. J. Biochem. Cell Biol. 2014, 51, 1–9. [Google Scholar] [CrossRef]
- Wu, C.-F.; Lin, Y.-S.; Lee, S.-C.; Chen, C.-Y.; Wu, M.-C.; Lin, J.-S. Effects of Davallia formosana Hayata Water and Alcohol Extracts on Osteoblastic MC3T3-E1 Cells. Phytother. Res. 2017, 31, 1349–1356. [Google Scholar] [CrossRef]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-β/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B.; Stein, J.L.; van Wijnen, A.J.; Frenkel, B.; Montecino, M. Mechanisms Regulating Osteoblast Proliferation and Differentiation. In Principles of Bone Biology; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Anter, J.; Quesada-Gómez, J.M.; Dorado, G.; Casado-Díaz, A. Effect of Hydroxytyrosol on Human Mesenchymal Stromal/Stem Cell Differentiation into Adipocytes and Osteoblasts. Arch. Med. Res. 2016, 47, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Hagiwara, K.; Shirai, N.; Yoshida, K.; Hagiwara, H. Apigenin inhibits osteoblastogenesis and osteoclastogenesis and prevents bone loss in ovariectomized mice. Cytotechnology 2015, 67, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Utsuyama, M.; Hirokawa, K. Expression profiles of receptor activator of nuclear factor kappaB ligand, receptor activator of nuclear factor kappaB, and osteoprotegerin messenger RNA in aged and ovariectomized rat bones. J. Bone Miner. Res. 2001, 16, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S. Minireview: The OPG/RANKL/RANK system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lin, D.; Qi, J.; Qiu, M.; Lv, Q.; Li, Q.; Lin, Z.; Liao, Z.; Pan, Y.; Jin, O.; et al. Serum from patients with ankylosing spondylitis can increase PPARD, fra-1, MMP7, OPG and RANKL expression in MG63 cells. Clinics 2015, 70, 738–742. [Google Scholar] [CrossRef]
- Satué, M.; del Mar Arriero, M.; Monjo, M.; Ramis, J.M. Quercitrin and taxifolin stimulate osteoblast differentiation in MC3T3-E1 cells and inhibit osteoclastogenesis in RAW 264.7 cells. Biochem. Pharmacol. 2013, 86, 1476–1486. [Google Scholar]
- Srivastava, S.; Bankar, R.; Roy, P. Assessment of the role of flavonoids for inducing osteoblast differentiation in isolated mouse bone marrow derived mesenchymal stem cells. Phytomedicine 2013, 20, 683–690. [Google Scholar] [CrossRef]
- Xiao, H.-H.; Gao, Q.-G.; Zhang, Y.; Wong, K.-C.; Dai, Y.; Yao, X.-S.; Wong, M.-S. Vanillic acid exerts oestrogen-like activities in osteoblast-like UMR 106 cells through MAP kinase (MEK/ERK)-mediated ER signaling pathway. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt B, 382–391. [Google Scholar] [CrossRef]
- Kim, M.-B.; Song, Y.; Hwang, J.-K. Kirenol stimulates osteoblast differentiation through activation of the BMP and Wnt/β-catenin signaling pathways in MC3T3-E1 cells. Fitoterapia 2014, 98, 59–65. [Google Scholar] [CrossRef]
- Xiao, H.-H.; Fung, C.-Y.; Mok, S.-K.; Wong, K.-C.; Ho, M.-X.; Wang, X.-L.; Yao, X.-S.; Wong, M.-S. Flavonoids from Herba epimedii selectively activate estrogen receptor alpha (ERα) and stimulate ER-dependent osteoblastic functions in UMR-106 cells. J. Steroid Biochem. Mol. Biol. 2014, 143, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kawaguchi, N.; Zaima, N.; Moriyama, T.; Fukuta, Y.; Shirasaka, N. Antiosteoporotic activity of a syringic acid diet in ovariectomized mice. J. Nat. Med. 2017, 71, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Abuohashish, H.M.; Khairy, D.A.; Abdelsalam, M.M.; Alsayyah, A.; Ahmed, M.M.; Al-Rejaie, S.S. In-vivo assessment of the osteo-protective effects of eugenol in alveolar bone tissues. Biomed. Pharmacother. 2018, 97, 1303–1310. [Google Scholar] [CrossRef] [PubMed]




| Gene | Sense Primer | Antisense Primer | Amplicon (bp) |
|---|---|---|---|
| TGFβ1 | 5’-TGAACCGGCCTTTCCTGCTTCTCATG-3´ | 5´-GCGGAAGTCAATGTACAGCTGCCGC-3´ | 152 |
| TGFβ- R1 | 5´-ACTGGCAGCTGTCATTGCTGGACCAG-3´ | 5´-CTGAGCCAGAACCTGACGTTGTCATATCA-3´ | 201 |
| TGFβ- R2 | 5´-GGCTCAACCACCAGGGCATCCAGAT-3´ | 5´-CTCCCCGAGAGCCTGTCCAGATGCT-3´ | 139 |
| TGFβ- R3 | 5´-ACCGTGATGGGCATTGCGTTTGCA-3´ | 5´-GTGCTCTGCGTGCTGCCGATGCTGT-3´ | 173 |
| RUNX-2 | 5´-TGGTTAATCTCCGCAGGTCAC-3´ | 5´-ACTGTGCTGAAGAGGCTGTTTG-3´ | 143 |
| OSX | 5´-TGCCTAGAAGCCCTGAGAAA-3´ | 5´-TTTAACTTGGGGCCTTGAGA-3´ | 205 |
| BMP2 | 5´-TCGAAATTCCCCGTGACCAG-3´ | 5´-CCACTTCCACCACGAATCCA-3´ | 142 |
| BMP7 | 5´-CTGGTCTTTGTCTGCAGTGG-3´ | 5´-GTACCCCTCAACAAGGCTTC-3´ | 202 |
| COL-I | 5´-AGAACTGGTACATCAGCAAG-3´ | 5´-GAGTTTACAGGAAGCAGACA-3´ | 471 |
| OSC | 5´-CCATGAGAGCCCTCACACTCC-3´ | 5´-GGTCAGCCAACTCGTCACAGTC-3´ | 258 |
| ALP | 5´-CCAACGTGGCTAAGAATGTCATC-3´ | 5´-TGGGCATTGGTGTTGTACGTC-3´ | 175 |
| RANKL | 5´-ATACCCTGATGAAAGGAGGA-3´ | 5´-GGGGCTCAATCTATATCTCG-3´ | 202 |
| OPG | 5´-ATGCAACACAGCACAACATA-3´ | 5´-GTTGCCGTTTTATCCTCTCT-3´ | 198 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melguizo-Rodríguez, L.; Manzano-Moreno, F.J.; Illescas-Montes, R.; Ramos-Torrecillas, J.; de Luna-Bertos, E.; Ruiz, C.; García-Martínez, O. Bone Protective Effect of Extra-Virgin Olive Oil Phenolic Compounds by Modulating Osteoblast Gene Expression. Nutrients 2019, 11, 1722. https://doi.org/10.3390/nu11081722
Melguizo-Rodríguez L, Manzano-Moreno FJ, Illescas-Montes R, Ramos-Torrecillas J, de Luna-Bertos E, Ruiz C, García-Martínez O. Bone Protective Effect of Extra-Virgin Olive Oil Phenolic Compounds by Modulating Osteoblast Gene Expression. Nutrients. 2019; 11(8):1722. https://doi.org/10.3390/nu11081722
Chicago/Turabian StyleMelguizo-Rodríguez, Lucía, Francisco Javier Manzano-Moreno, Rebeca Illescas-Montes, Javier Ramos-Torrecillas, Elvira de Luna-Bertos, Concepción Ruiz, and Olga García-Martínez. 2019. "Bone Protective Effect of Extra-Virgin Olive Oil Phenolic Compounds by Modulating Osteoblast Gene Expression" Nutrients 11, no. 8: 1722. https://doi.org/10.3390/nu11081722